Huntington’s disease therapeutics conference 2023 - Day 2
Check out research updates from Day 2 of the 2022 HD Therapeutics Conference #HDTC2023

 By Joel Stanton, Dr Rachel Harding, Dr Leora Fox, and Dr Tamara Maiuri May 04, 2023 Edited by Dr Sarah Hernandez Originally published on April 27, 2023
By Joel Stanton, Dr Rachel Harding, Dr Leora Fox, and Dr Tamara Maiuri May 04, 2023 Edited by Dr Sarah Hernandez Originally published on April 27, 2023
Welcome to the second full day of HD science, live from Dubrovnik! After yesterday’s amazing basic science talks, today begins with a session focused on companies developing new experimental treatments for HD.
Our Twitter updates are compiled below. Continue to follow live updates for the rest of the conference with the hashtag #HDTC2023.
Check out our coverage of Day 1 here: https://en.hdbuzz.net/343 . We’ll post summaries in article format for each day of the conference.
HD therapeutic candidates
A unique approach to ASOs
The first speaker of the morning is Dr. Nicole Datson, from a company called VICO - they work with a type of drug familiar to many HD families, Antisense Oligonucleotides, or ASOs. Several companies, including Wave Life Sciences and Roche, are already testing ASO drugs in HD, but Vico’s approach is unique. VICO’s ASO directly targets the genetic mutation that causes HD - an expanded “CAG” sequence near the beginning of the huntingtin gene. This has very interesting implications, because HD is not the only “CAG repeat expansion” disease - at least 8 other human diseases are caused by the same exact genetic change, but in different genes across the genome. So, if the drug works, it could potentially be applied to any one of this family of diseases that are associated with the same genetic change.
Dr. Nicole Datson, Vico’s Chief Scientific Officer, gives an overview of Vico’s approach. Even the regular huntingtin gene has a long CAG sequence (~17-20 in most people without HD), so it’s difficult for these drugs to specifically bind only the disease form of huntingtin. But Vico suggests that while their ASOs targeting CAG don’t only recognize the longer form that causes the disease, it seems to prefer these longer CAGs, so there’s more impact of the drug on the expanded CAG repeat. Nicole is showing their data from HD patient cells after treatment with their ASO drug, snappily called VO659. Treatment with higher doses of the ASO leads to greater reduction of the expanded huntingtin protein, the bad guy of HD.
Excitingly, the same ASO also has effects in cells from patients with two other brain diseases involving CAG expansion, forms of ataxia called SCA1 and SCA3. In both cases, the ASO also prefers the mutant form of the gene with the longer CAG repeats. If it works, the cool implication of this is that a single drug could potentially work for a whole alphabet soup of diseases caused by CAG repeat expansions including HD, SCA1, SCA3, DRPLA, and SBMA.
Moving away from cells, Nicole shows that treating HD mice with ASO also leads to reductions in the huntingtin protein. The behavior and brain anatomy of the HD mice was also improved by the ASO treatment - very cool. Vico’s data shows that the ASO stays in the brains of animals for a long time, suggesting that they might be able to have fairly large intervals between treatments. This would be a big benefit for ASOs that require injections into the spine to reach the brain.
In mouse models of two other CAG-expansion diseases, SCA1 and SCA3, they saw similar benefits: the disease versions of the gene were switched off to a greater degree, leading to improvements in disease-like symptoms. VICO have also tested their drug in monkeys and saw that their drug spreads fairly well throughout different regions of the brain and stays in the brain for a long time, same as they saw in the mice. Based on their animal data, Vico thinks that for both HD and the other diseases they could inject their drug as infrequently as a couple times per year. This would make treatment much easier on families, making it easier to participate in the trial. Given these promising animal and cell studies, Vico launched an early stage human study in patients with HD, SCA1, and SCA3 - very cool they’re targeting these conditions all in one “basket” trial.
RNA interference
Up next is Dr. William Cantley, a researcher at Alnylam Pharmaceuticals, a leading company in the area of “RNA interference”, also called RNAi. This is a totally distinct chemistry from ASOs, but has a very similar goal - to reduce levels of a target protein. In theory RNAi is more potent than ASOs, meaning you need less drug for the same effect, but to date ASOs are being widely used in brain diseases. Part of the problem is delivery - how to get RNAi drugs to the 84 billion brain cells that might need to get treated in HD. Getting RNAi drugs into other cell types, such as the liver, has been improved by sticking little molecular keys onto RNAi drugs. When cells in the body have a matching lock on their cell surface, they can take up RNAi drugs much more easily. Alnylam has a lot of experience with these lock-and-key tricks to get RNAi drugs into liver cells. But treating the liver is less important in HD. So Alnylam has developed a new key, “C16,” that unlocks important brain cell types that we care about in HD.
William shows very cool data in another brain disease, Alzheimer’s, in which tagging an RNAi drug with C16 seems to work very well to get into the brains of monkeys. William is announcing - for the first time publicly - that Alnylam is working on an RNAi drug for HD using this cool new C16 key approach.
After walking the crowd through Alnylam’s strategic decision process, William gives an overview of some early work in HD mice with a new C16-RNAi drug. Using two different drugs, targeting different parts of the HD gene, they see reduction of huntingtin protein. In addition to these HD mouse studies, Alnylam also conducted studies in monkey brains which show very big reductions of HTT protein levels in the cortex, the wrinkly outside part of the brain, that’s affected HD. Exciting to have another arrow in the quiver of HTT lowering drugs, as RNAi drugs and ASO drugs have different risks and benefits. We won’t know until we’re done which of these approaches provides the most benefits, so great to have another runner in the race.
Making a huntingtin protein degrader
The final talk before the first coffee break of the day is from Adam Hendricson, who works at Arvinas Operations. Adam will be telling us about their work to make a huntingtin protein degrader - a small molecule drug which can reduce levels of huntingtin by sending it to the cell’s rubbish bin.
In recent years, there has been a scientific explosion in a new kind of technology focused on breaking down disease proteins with small molecules called PROTACs. This is exciting as these drugs COULD be taken by mouth if they work out. PROTACs work by bringing a rubbish-labeling protein in our cells into close contact with a target protein, in our case the huntingtin protein. This would lead to huntingtin being tagged in a special way that tells the cell to treat it like trash. Arvinas is one of the first companies to bring this technology into a clinical trial. They are currently testing one of their drugs to treat cancer, showing it is possible for this new approach to be more than just a cool tool for lab scientists. They are working on a number of different brain diseases including Alzheimer’s, Parkinson’s, and HD.
Arvinas are hoping to target the “soluble” huntingtin protein, the form that precedes the formation of the more solidly structured protein clumps. They are also looking for drug molecules which have preference for mutant over normal huntingtin protein. In cells in a dish, they show that the PROTAC they have identified binds both the mutant huntingtin protein, and the trash-labelling protein. These molecules bind very tightly which is what drug-hunters like Adam are looking for!
The PROTAC molecule from Arvinas can in fact lower the levels of both the soluble and clumped form of the expanded huntingtin which is good news, as scientists think reducing levels of both of these proteins would be beneficial in patients. Most of this work so far has been experiments in cells in a dish but Arvinas are now starting to test their molecule in mice. The good news is that it looks like the PROTAC is able to get into the brain, which is often very challenging for drugs to do.
Tracking the superhumans
Next up we’re hearing from Dr. Donna Finch of Alchemab Therapeutics. Their focus is targeting the immune system, using antibodies that could potentially be protective to brain cells in HD. Their approach is to find naturally occurring protective antibodies in patients who are resilient i.e. very long-lived or less affected by disease genes. Then they do all sorts of screens to figure out why their antibodies are protective, and go on to test the best ones in cells and in animals.
This is like tracking down the natural “super humans” among us and working out why disease seems to affect them less than most people, and using that information to help treat others. Alchemab has come to this conference to find out how they might use their approach for HD - very exciting to have another company come to work on a new avenue of therapeutics! They collaborate with organizations across the world that have helped them to gather samples from people with different diseases and vulnerabilities. They have compiled immune system info into a “data cube” that allows them to do large scale analysis across many individuals.
We are living in the age of BIG data! Companies like Alchemab use absolutely huge data sets and lots of clever computer tools to find small signals in the data which could uncover new biology. They’ve found that antibodies against the huntingtin protein are generated in resilient individuals, even in other diseases like Alzheimer’s. An antibody has been identified by Alchemab, called ATLX-1095, which binds to a fragment of the huntingtin protein. The idea is that this antibody could tell the immune system to focus on clearing away the harmful protein.
In early assays this antibody prevents pieces of harmful huntingtin from sticking together and starting to form larger clumps which are thought to be toxic. Alchemab work with a number of academic collaborators who are now repeating these experiments - it’s a cool partnership! Alchemab has also shown that ATLX-1095 increases the amount of huntingtin cleanup being performed by a type of support cell called microglia. So the drug is helping to “gobble up” trash in cells, as Donna puts it. The drug is also able to penetrate into the brain. While these antibodies originally come directly from human cells, they need to be manufactured on a larger scale in order to do more experiments and eventually, human trials. Alchemab has had early success in being able to produce the antibody in a laboratory. Now they are testing this potential drug in mice, so we will watch this space as they progress through these next stages of preclinical work!
A new way to trash harmful huntingtin
Next up is Dr. Pavlina Konstantinova who is from VectorY. They work on a number of different brain diseases, including ALS, Parkinson’s, and HD. VectorY, like many other companies we’ve heard from, are trying to specifically target the mutant copy of the huntingtin protein while sparing the normal copy. They are specifically focused on targeting the part of the protein that arises from the CAG repeat expansion, which occurs right at the beginning of the gene. This encodes a long string of glutamines which is what makes this protein sticky and clumpy.
VectorY is also developing a new technique known as VecTron, similar to PROTAC that we heard about earlier. It’s another way of putting a “trash tag” on the harmful huntingtin protein that tells the cell to break it down and throw it away. Pavlina is sharing data showing that these “VecTrons” reduce the amount of huntingtin clumps found inside cells in a dish. They have also treated HD mice, and so far they are seeing reduced huntingtin clumps as well as some improvement in their movement symptoms.
They are also working on a treatment for ALS using delivery with viruses, and have tested its ability to spread through the brain in mice and larger animals like pigs and primates. So far they are seeing good spread, meaning that the treatment reaches many areas of the brain. VectorY is also working on improving the technologies needed to direct their targets for clearance by the cell, to deliver the drugs to the right parts of the brain, and to manufacture the drugs on the scale needed to bring it to patients.
Targeting MSH3
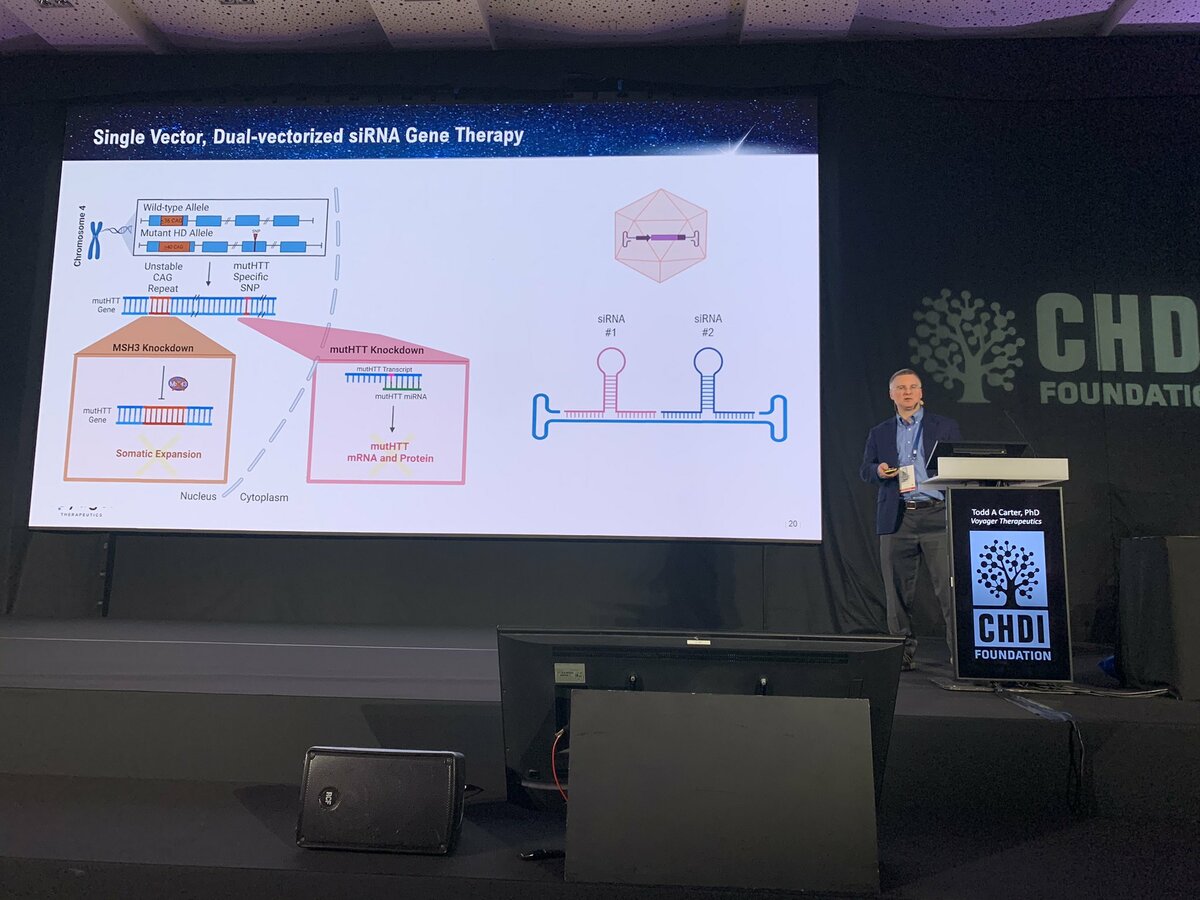
The final talk before a break for lunch is from Dr. Todd Carter at Voyager Therapeutics - that’s right, yet another company working on HD, very exciting! Voyager is working on targeting MSH3, the genetic modifier we heard a lot about yesterday. Voyager is a “gene therapy” company - meaning they focus on delivering therapies to the body’s cells using harmless virus particles. Voyager’s particular focus is a type of virus called AAV that’s naturally really good at breaking into brain cells, the most important target for HD.
Todd describes that Voyager’s new viral particles, which they call TRACER AAV, have a very unique capability. Unlike natural AAV’s, this engineered virus is able to jump into the brain after being injected into the bloodstream. Most AAV’s are totally stopped by the blood brain barrier (BBB), a tight seal that protects the brain from harmful things like viruses and other toxic molecules found occasionally in the blood. Most AAV’s, if injected in the blood, are totally excluded from the brain.
Using clever technology, Voyager has constructed AAVs that can make their way through the BBB into the brain after a simple injection in the blood. If this works, it could simplify gene therapy for HD compared to previous approaches which relied on direct brain injections. Compared to natural viruses, Voyager’s new AAVs get to a larger portion of the brains of monkeys when they tested them out. This suggests that there’s a good chance that this might provide a way to deliver useful payloads to the brains of people with HD. One cool feature of this technology is it could be used to carry multiple therapeutics for delivery into the brain at the same time. For example, huntingtin lowering and MSH3 targeting drugs which could be used in a combination treatment for people with HD.
Biomarkers and HD: the keynote speech
After a short break we are back for the keynote speaker this afternoon. The talk will be from Dr. Henrik Zetterberg, who is affiliated with University of Gothenburg and University College London. This talk will be focussed on biomarkers for HD, including neurofilament light, also called NfL.
Henrik is starting with a history of the people, the technology, and the discoveries that led us to the use of NfL as a biomarker - something we can reliably measure to track the progression of HD as well as other brain diseases. Work from teams at University College London, including HDBuzz’s own Prof. Ed Wild, has shown that there is a relationship between levels of NfL and aspects of HD, such as CAG repeat length and timing of symptom onset. NfL can be used as a marker of brain damage in injury and disease; for example Henrik has shown increased levels of NfL in boxers right after they spar, especially when they take many hits. NfL levels also drop after successful treatment of nervous system diseases like multiple sclerosis, spinal muscular atrophy, HIV, and more recently forms of Batten disease and Alzheimer’s. Researchers can measure NfL in biofluids like blood and CSF (the fluid that bathes the brain and spinal cord). In some diseases, like HD and Alzheimer’s, NfL levels go up just as symptoms begin to appear.
Henrik’s lab is also using NfL to try and tease out whether a person experiencing new symptoms might have a psychiatric disease, like major depression or schizophrenia (low NfL), versus a neurodegenerative disease, like HD or Alzheimer’s (high NfL). Another area in which NfL measurement could be useful is to help doctors understand whether someone is experiencing neurological injury or unexpected side effects following general anesthesia, cancer treatment, or surgery. New methods are making it easier and easier to measure NfL - rather than having to take a sample of spinal fluid and preserve it carefully for analysis, sample collection could be done at home with a finger prick! He’s also looking at NfL from some unexpected and rather wacky angles - like showing that the brain’s support cells “eat” NfL, showing it goes up in bears when they hibernate, or that it increases in astronauts who go to space! Overall, Henrik emphasizes that NfL can be useful as a measurement of disease onset, as a way to understand the safety of a drug or a procedure, and as another angle to observe other factors that influence HD symptoms, like age and genetic variations.
That’s all for day 2! We will be back tomorrow morning to report on the third and final day of HDTC2023! Remember to continue to follow live updates for the rest of the conference on Twitter, with the hashtag #HDTC2023.


