Exciting new Huntingtin lowering tool described
Exciting new Huntingtin Lowering work from @SangamoTx and @CHDIfoundation using "Zinc Fingers" to shut down expression of the mutant Huntingtin gene. More details on this exciting new technique here.

An exciting new tool in the fight against Huntington’s disease has just been described. An international group of scientists have developed a new, targeted, way to lower levels of the mutant huntingtin protein.
Huntingtin genetics: from gene to protein
Huntington’s disease (HD) is caused by a genetic change – or mutation – in the DNA of a specific gene. Scientists call the gene huntingtin. Like every other bit of DNA in our cells, the huntingtin gene is comprised of four chemical letters, which repeat in unique patterns that give them their unique functions.
Those four DNA letters are referred to by abbreviations for their chemical names, ‘A’, ‘C’, ‘T’, and ‘G’. Every case of HD is caused by a lengthening of a long stretch of the DNA letters ‘C-A-G’ very near the beginning of the huntingtin gene. In most people – the ones not destined to develop HD – that ‘C-A-G’ code is repeated around 20 times or so, for reasons we still don’t totally understand.
HD arises when a person inherits a lengthened stretch of ‘C-A-G’, with the disease inevitably arising in people who inherit 40 or more ‘C-A-G’s. Note that everyone has two different copies of the huntingtin gene – one inherited from Mom and one from Dad. The vast majority of HD patients have a normal copy with a low number of ‘C-A-G’s, and the mutant copy in which they are longer.
Most genes, including the huntingtin gene, are used by cells as instruction manuals for building proteins – tiny molecular machines that help cells do their work. So in the cells of people with the HD mutation, there’s two different versions of the huntingtin gene, and those instructions tell the cell to make two different versions of the huntingtin protein.
Huntingtin Lowering
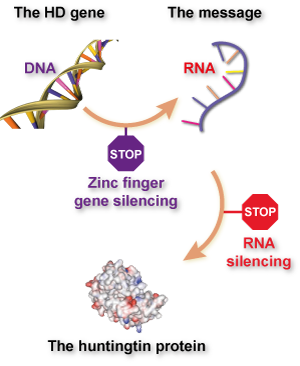
A major goal of the HD research world currently is to investigate whether “huntingtin lowering” strategies could be effective treatments for HD. The goal of huntingtin lowering treatments is to stop, or slow, the rate at which cells use the information in the huntingtin gene to make the huntingtin protein.
Animal studies suggest that if we can lower the amount of huntingtin protein made from the mutant huntingtin gene, we may have a hope of reducing the symptoms of HD. A number of drug companies are using a wide range of approaches to lower huntingtin as potentially new treatments for HD. We’ve covered the general idea of huntingtin lowering here, with more recent updates about huntingtin lowering drugs called ASOs here and here, and other approaches here and here.
And now, ZFPs
The biotechnology company Sangamo Therapeutics has been working for a number of years on yet another way of lowering proteins: by controlling whether a gene gets turned on, or activated. Their technology relies on little molecular machines called zinc finger protein transcription factors. That’s kind of a mouthful, so we’ll just call them ZFPs for short. Just like the other huntingtin lowering technologies we’ve described before, the goal for researchers using ZFPs in HD is to reduce huntingtin levels in cells.
While the basic idea is the same, ZFPs work in a quite unique way, compared to existing huntingtin lowering technologies. Existing huntingtin lowering drugs work by targeting an intermediate step between reading the huntingtin gene’s information from DNA and making the huntingtin protein. The information in genes is first read from the DNA, copied into a closely related language called RNA and then translated into the language of proteins. This intermediate RNA message is the target of huntingtin lowering drugs currently in the clinic.
But ZFPs, like those developed by Sangamo and their collaborators, work in a very different way. Our cells contain a number of proteins that include tiny little pincers, which are shaped just right for grasping specific DNA sequences. (Nerd alert – the pincers are held together by a zinc atom, which explains the funny name).
“Unlike approaches that target the huntingtin RNA, cells treated with ZFPs never turn on their huntingtin gene in the first place.”
ZFPs for HD?
For many years, researchers have worked towards understanding naturally occurring ZFPs in the hopes that they could reprogram them to stick to new specific DNA sequences. Sangamo have been a leader in this field, and developed a sort of tool kit of custom ZFPs that can target almost any DNA sequence.
Why do this, what’s the point of making custom DNA-binding pincers? Well, it turns out that we can attach various payloads to these pincers, and some of them do very interesting things to the DNA where they attach. As an example, researchers know that they can fuse a sort of cellular stop sign to zinc fingers, to block the cell from activating the targeted gene.
A recent publication describes Sangamo’s work developing ZFPs for use in HD, which was a large-scale collaboration with CHDI foundation and a number of HD researchers around the world. After a laborious screening effort, they were able to develop new ZFPs that stick to the huntingtin gene – in the DNA – and block its activation. So, unlike other approaches that target the huntingtin RNA, cells treated with these ZFPs never turn on their huntingtin gene in the first place.
Even better, the team was able to develop ZFPs that can shut off expression of only the mutant copy of the huntingtin gene, while leaving the normal copy entirely alone. Sangamo tested their ability to discriminate between one of the lowest CAG sizes that cause HD in humans (38 CAG repeats), while leaving the normal copy of huntingtin alone.
Promising results in mice
Having proved in cells that their new ZFPs could shut off mutant huntingtin specifically, the team next did a number of very well-conducted animal studies to see if their tool might be useful in the brains of animals that have HD-like mutations. To be comprehensive, they tested two different animal models of HD – one with very rapidly progressing symptoms, and another with more subtle long-term changes.
In both cases, ZFP delivery to the brains of mice led to reductions of the huntingtin protein. It also helped some of the symptoms these mice experience, which look a bit like things we observe in HD patients.
It’s reasonably easy to test experimental drugs like this in mice. Researchers are able to collect brain tissue from animals and study it intensively, but similar studies are impossible in human HD patients, who get quite grumpy if you take pieces of their brain. Because translating mouse studies into humans is so difficult, the team did another set of experiments to determine whether ZFP treatment improved things in a way that we can also measure in people.
In fact, using sophisticated brain scanning techniques, the team was able to observe benefits of ZFP treatment in HD mice. These well-established techniques also work in humans, so if we want to test ZFPs in human studies we can hope to look for improvements without the need for removing tissue.

What are the risks and benefits of ZFPs?
As with every other potential treatment for HD, there are benefits and disadvantages to the use of ZFPs. In theory, it’s a much better approach to shut off the protein production from a mutant gene entirely, rather than trying to clean up the RNA and protein afterwards. We don’t completely understand which RNA and protein species have toxic effects in cells, so shutting it off at the tap seems like the best approach.
Moreover, the data presented by Sangamo and their collaborators shows a very nice ability to discriminate between the normal copy of the huntingtin gene and the mutant copy. Silencing just the mutant copy of the huntingtin gene and sparing the other copy is, in theory, preferable, since we still don’t know every risk associated with reducing the normal copy.
On the downside, the ZFPs developed by Sangamo and their collaborators are genes themselves, encoded in DNA, that must be delivered to every cell we want to treat. Using delivery of genes to treat a disease is generally known as gene therapy. To be an effective treatment for HD, ZFP gene therapy will require certain interventions. The DNA encoding the ZFPs needs to be packaged up into a virus and injected into the brain.
Like any drug, the ZFPs developed by Sangamo and their collaborators could have unexpected consequences. In this case, the simplest concern about ZFPs might be that they accidentally target other genes – besides huntingtin – for lowering. The team conducted quite detailed investigations of this possibility in cells, but of course in the brain things could be more complicated.
The best way to determine whether these ZFPs are as useful as we would hope is to run human studies. To support this, Sangamo has established a partnership with Japanese drug giant Takeda, who certainly have the expertise and resources to run such studies. Stay tuned to HDBuzz for any announcements about future studies with ZFPs in HD patients.
Take home
This exciting new study provides another arrow in our quiver as we tackle huntingtin lowering in the clinic. The study was very well-conducted, and leaves us well-poised to consider testing ZFPs in human clinical studies. It’s very exciting to see that brilliant scientists around the world continue to develop new approaches to treating HD.
These new ZFPs seem likely to provide exciting benefits compared to other Huntingtin lowering approaches that we look forward to seeing tested in HD patients. Stay tuned to HDBuzz for more coverage of huntingtin lowering therapies!
Learn more
For more information about our disclosure policy see our FAQ…


