Details emerge of first Huntington’s disease gene therapy clinical trial
UniQure announces key details of its planned trial to assess the safety and ability of AMT-130 gene therapy to lower the problematic huntingtin protein using a ‘single-shot’ virus delivery system.
At the recent Huntington’s Disease Society of America annual convention in Boston, UniQure announced crucial details of its planned clinical trial for its experimental therapy, AMT-130. We previously wrote about AMT-130 here, so this article covers the basics and what’s just been announced.
Huntingtin-lowering gene therapy in an nutshell
AMT-130 is a huntingtin-lowering treatment, because it seeks to reduce production of the harmful mutant huntingtin protein, which is harmful to neurons and is the cause of Huntington’s disease.

However, AMT-130 differs in several important ways from the anti-sense oligonucleotide (ASO) trials that are currently underway, run by Roche and Wave Life Sciences.
AMT-130 is a gene therapy, which means that it seeks to permanently change the genetic makeup of the treated patient. AMT130 doesn’t try to delete the HD mutation – that is much harder to achieve than you might think. Instead, AMT130 utilizes a harmless virus known as an adeno-associated virus (AAV) to add a small extra piece of genetic code to neurons. That code is a set of instructions for making a huntingtin-lowering drug.
Once a neuron is treated with AMT-130, it will continuously manufacture additional copies of the new Huntingtin-lowering molecule. So while the neuron still contains the harmful HD gene, and still sends messages to make the mutant huntingtin protein, at the same time it will be producing a new set instructions to delete the huntingtin message. The result should be reduced production of the harmful protein, with a very long duration of effect – possibly lifelong.
What about the trial?
UniQure announced some preliminary but important details about its planned trial in a statement to the HD community. Here’s what we know so far.
The focus of the first trial will be safety and tolerability – finding out whether there any harmful or unpleasant effects of receiving AMT-130 treatment.
UniQure also includes efficacy in the stated aims of the study: that means getting an idea of whether the treatment is doing what it’s supposed to do. In the broader sense, that means slowing the progression of Huntington’s disease. It’s theoretically possible, but very unlikely, that this small first trial will show evidence of slowed progression. A more achievable aim is to test whether treatment reduces huntingtin production, which we can now measure using techniques we’ve described on HDBuzz here.
The AMT-130 trial will be based at HD clinical sites in the Unites States. We don’t know what sites or how many, yet. These will be publicly announced when they come online. UniQure hopes to begin enrolling patients before the end of 2019.
The trial will enrol just 26 patients with early symptoms of Huntington’s disease. That means people with abnormal movements, within the first few years after diagnosis was ‘officially’ confirmed by a neurologist. The age range is 25 to 65 years of age.
Unusually, uniQure has set a cutoff of 44 CAG repeats or more in the HD gene. About 50% of people with a positive genetic test for HD have between 40 and 45 repeats, so this cutoff may well exclude quite a few people. It’s likely that uniQure set this cutoff to skew the trial towards people likely to progress more quickly, so that they can get a better chance of showing that AMT-130 slows progression.
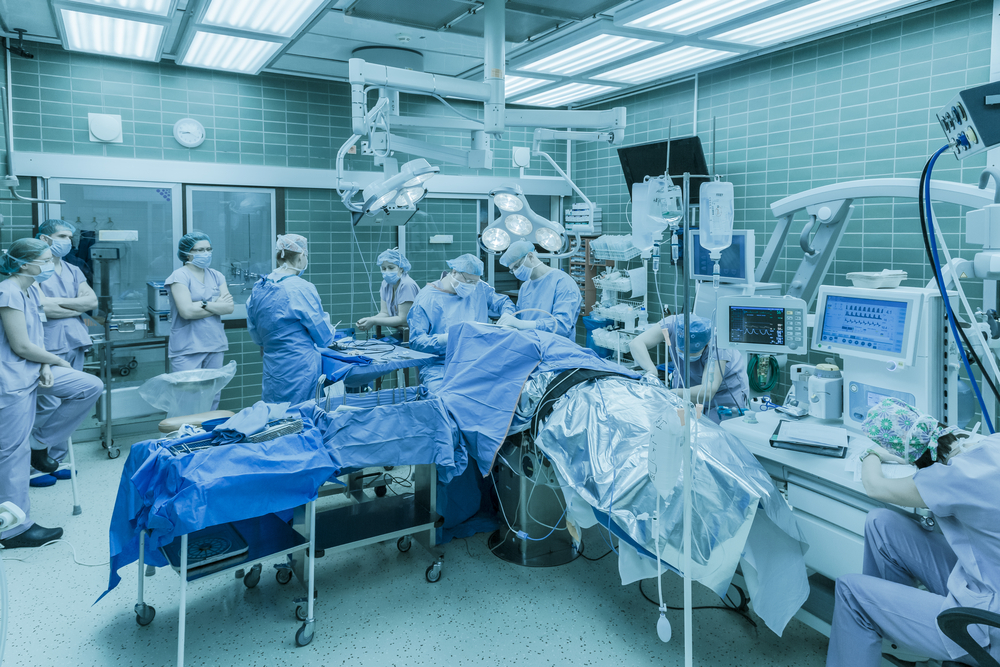
The 26 patients will be split at random into two groups. 16 patients will receive active treatment with AMT-130, at either a low dose or a high dose. 10 patients will undergo “imitation” treatment, which could also be referred to as “placebo” or “sham” treatment.
The potentially big benefit of treatment with AMT-130 is that it’s only performed once per participant, but it’s a big deal. It involves brain surgery, carried out under general anaesthesia. Between two and six small holes will be drilled in the skull, and thin tubes called catheters inserted into the brain. The AMT-130 cocktail is then injected down the tubes into the brain.
Patients assigned to the imitation treatment will undergo general anaesthesia, and shallow holes will be drilled into the outer layer of the skull but won’t pass right through it. No tubes or injections are involved.
The purpose of the imitation group is to help figure out whether any effects seen in the trial – be they helpful or harmful – are caused by the AMT-130 therapy itself, the placebo effect (the psychological boost that comes from being in a clinical trial), or the effects of undergoing anaesthesia and surgery.
Patients will be followed up intensively for 18 months, with investigations including MRI scans and lumbar punctures (spinal taps). Patients in the active group will then be asked to return for annual visits for 5 years.
The neurosurgical team will know which patients are in which group, but the patient and the HD trial team will not know. This means the study is double-blinded and helps minimize the placebo effect, enabling the trial to deliver on its scientific aim of figuring out whether the drug is safe and whether it works.
The upside for patients assigned to the imitation group is that 18 months after their mini-operation, if an independent review of the trial data is satisfactory, they will be invited to undergo full treatment with AMT-130 in a second, bigger operation.
Risk and reward
The main potential advantage of AMT-130 is also its biggest potential drawback. It’s a gene therapy, so a single treatment is expected to be permanent.
If everything works out as planned, it will be a treatment that could be given early after a positive genetic test result, that will have long-lasting, possibly lifelong benefits. It could slow progression or even delay the onset of HD without requiring repeated dosing.
However, if the treatment turns out to have harmful side-effects, these could be long-lasting too – and there is no way to switch off the treatment once it’s been given. As a purely fictional example, what if the treatment for some reason causes a worsening of movement control, an acceleration of the person’s Huntington’s disease, or a permanent feeling of nausea? Patients might be left with major symptoms or even disabilities. The medical team would do everything they could to improve things, but removing the treatment or switching it off won’t be an option.
Another important detail is that AMT-130 is designed to reduce production of both forms of the huntingtin protein – not just the harmful mutant form, but its innocent ‘normal’ sibling, which scientists call ‘wild-type’. One concern is that reducing wild-type huntingtin in the brain of adults with HD might come with risks of its own, which could eclipse any benefits from lowering the mutant protein. These concerns come largely from experiments carried out in mice, which all have major differences from human patients, and at the moment the actual effects of lowering wild-type huntingtin in patients are unknown. Important insights on this will hopefully come from the two ongoing huntingtin-lowering programs involving drug injections into the spine: Roche’s RG6042, which lowers both forms of the protein, and Wave’s Precision-HD program, which is attempting to lower the mutant form selectively.
AMT-130 has been tested in animals, and would not have been permitted to proceed to a human trial if unacceptable risks had been discovered. But only humans can tell us what benefits and harms might be seen. On top of the risks of surgery, patients considering volunteering in this trial can expect to be thoroughly counselled about the possible risks, without any firm expectation that they will benefit personally from taking part. Such volunteers will be asked to undertake significant sacrifice on behalf of others – those that volunteer for first-in-human trials are among the HD community’s greatest individual heroes.
An important advance
Here at HDBuzz, our favourite cocktail is called Substantive Hope. It’s equal parts optimism and realism. After our previous reporting on huntingtin-lowering therapies, we’ve received some feedback that we’re being too positive, and some that we’re being too negative (the word “HDBuzzKill” has even been bandied about, and we’re OK with that). Perhaps this means we’re getting it about right – but it’s up to you to decide.
We encourage all readers to get their information from multiple sources. Our ‘Ten Golden Rules’ article, published in 2011, might help you cultivate a mind that’s open to cool new ideas but healthily skeptical towards hype. We are unashamedly in favour of HD family members volunteering for research projects including clinical trials: this is the only way we will ever make real progress in the fight against Huntington’s disease. We encourage anyone thinking of volunteering to weigh the risks and benefits and seek advice from smart people you trust, before signing up.
Our take on AMT-130: the first huntingtin-lowering gene therapy trial has the potential to pave the way for a new generation of truly revolutionary drugs. Those taking part are no less brave than the astronauts who stepped onto the surface of the moon almost exactly fifty years ago. At significant personal risk, they will volunteer to take a not-so-small step into the unknown, in the hope of producing a giant leap for HD families.
We’ll publish further updates about this and other huntingtin-lowering programs as they develop.
Learn more
Sources & References
For more information about our disclosure policy see our FAQ…