Gene silencing for HD: the story so far
Is gene silencing as exciting as it sounds for HD, and what might the future hold?

Gene silencing means using specially designed molecules to ‘switch off’ the message that makes cells produce the harmful huntingtin protein. Our HDBuzz gene silencing primer explains the techniques, results so far and the challenges ahead.
We’ve had lots of requests from readers to write an article on ‘gene silencing’ – also known as ‘huntingtin silencing’. It’s an area of research that generates a lot of excitement. Many people’s attention has been drawn to the topic by the recent joint press release from Lundbeck and the University of Massachusetts, announcing a research collaboration aimed at developing ‘RNA interference (RNAi)’ therapies for HD.
So, what is gene silencing? How does it work, and how might it benefit HD patients?

Genes, messages and proteins
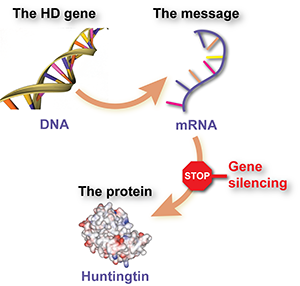
Proteins are molecules that act like tiny machines, carrying out most of the useful work inside cells – things like making chemical reactions work, communicating messages, giving cells their structure and so on. Each different protein is made using a set of instructions called a gene. Genes are made from DNA, and live in the control center of the cell, the nucleus.
Genes aren’t used to make proteins directly, though. In between, the cell uses the DNA sequence of the gene as a template to make a ‘message molecule’ called ‘messenger RNA’ or mRNA. The mRNA message molecule is then used to tell the cell what building blocks to stick together to make the protein.
To recap, DNA is used as a template to make an mRNA message, and the message molecule is then used to build protein molecules.
Huntington’s disease is caused by a single abnormal gene – the gene that tells cells to produce the protein called huntingtin. Each cell has two copies of every gene. Most people with HD, or those who will develop it later, have one ‘normal’ gene and one with too many repeats of the three-letter sequence ‘CAG’ near the beginning. That simple ‘spelling mistake’ results in a ‘mutant’ protein that behaves differently from the normal protein, damaging cells and producing the symptoms of HD.
You might have heard the term ‘wild-type‘ – that’s what scientists call the non-mutant, or ‘normal’, gene and protein.
Silencing the huntingtin gene
Since the abnormal gene is the cause of all the problems in HD, why not just get rid of it and replace it with a healthy gene? Unfortunately that’s unlikely to work, because cells have very secure ways of protecting the DNA to prevent damage or alterations.
The mRNA message molecule, on the other hand, is floating about in the cell, and as long as it’s there, it will keep being used to make more protein molecules. If we could somehow tell the cell to ignore that message, the harmful protein wouldn’t get made. That’s the thinking behind ‘gene silencing‘. The idea is that scientists could create a drug that is actually a specially designed message molecule, that sticks to the huntingtin message and tells the cell to get rid of it.
Gene silencing sounds too good to be true, but it isn’t. In 1998, two researchers, who went on to win the Nobel Prize for Medicine, worked out how to switch off individual genes. They called their technique RNA interference (RNAi).
Gene silencing is now a standard technique used by scientists to study how organisms work, how diseases cause damage and as a way of developing treatments. One gene silencing drug (Vitravene, used to treat a viral eye infection) is licensed for human use, and over a dozen trials are ongoing in many different diseases, with more on the way.
If it was as easy as that, we’d have gene silencing pills for Huntington’s disease already, so what’s the catch? Well, as with any new technique there are bound to be challenges, setbacks and unexpected hurdles along the way.
Challenge one: getting into the brain
One of the major problems is getting the silencing molecules to where they’re needed. In HD, death of brain cells called neurons is the major issue, so we need to get the molecule into those cells.
“Several different groups of researchers have now reported success in lowering huntingtin production in animal models”
The first hurdle is getting the drug into the brain. The brain has a natural defensive shield to prevent harmful substances entering it from the blood. That’s good for us overall, because it protects the brain, but it gives HD drug researchers a headache, because it make it much harder to get drugs into the brain than into, say, the liver or kidneys.
So it’s unlikely that a simple tablet or injection would be suitable for fighting HD with gene silencing. One way around this problem is to use pumps and tiny tubes to infuse the silencing drug directly into either the brain or the fluid that surrounds it – cerebrospinal fluid or CSF. That sounds pretty daunting, and there’s no doubt that implanting pumps or tubes into the nervous system is a big deal, but actually similar systems are already used to deliver drugs in other diseases such as multiple sclerosis (MS) and brain cancer, where they have a very good safety record.
Challenge two: distribution
Once the drug is in the nervous system, the distribution problem still isn’t solved. The brain is a dense ball of tissue which it’s difficult for the silencing molecules to spread through. Moreover, the treatment needs to get inside the cells to work – floating around between cells isn’t enough.
Scientists are using different methods to crack this problem. The Nobel Prize winners used RNA interference molecules (RNAi) to switch off genes. Those are very similar to molecules produced naturally by cells. The disadvantage is that they don’t tend to spread naturally through the brain, and aren’t terribly good at getting into cells.
So, RNAi researchers tend to use very fine tubes, inserted into the substance of the brain, targeting the worst-affected regions, connected to pumps that use pressure to spread the drug further. Another option is to let the RNAi molecules ‘hitch a ride’ inside deactivated viruses, which are experts in spreading round the brain and injecting stuff into cells.
Another approach is to try different molecules that might be better at spreading around and entering cells. Antisense oligonucleotides (ASOs) are similar to RNAi molecules but are slightly simpler and aren’t produced naturally by cells. The principle is the same – they stick to the mRNA message molecule and prevent the cell from using it to build proteins.
ASOs seem to be much better at spreading throughout the brain and can enter cells quite easily. They also seem to last much longer – which could be a good thing or bad, depending on how well they do their job.
Which gene silencing technique is better? We just don’t know, so RNAi and ASOs are being worked on at the same time, to see which is best.
Challenge three: switching off the gene
The key test of a gene silencing treatment is whether it can switch off the gene successfully. So far, research in HD animal models has strongly suggested that this can be achieved, with both RNAi and ASO treatments.
In 2005, a team of researchers led by Bev Davidson in Iowa injected RNAi molecules into the brains of HD mice, and achieved an 85% reduction in the huntingtin message. The motor function and cellular abnormalities of the mice improved, too. Since then, several different groups of researchers have reported success in lowering huntingtin production using various different molecules, including RNAi and ASO drugs. The most recent work suggests that the benefits of even short infusions are sustained for quite long periods.
Challenge four: choosing the right target
Drug researchers like to spot problems in advance rather than wait for them to happen, and one possible problem with gene silencing is its effects on the natural or wild-type copy of the huntingtin gene.
We know that having no huntingtin at all is dangerous. Mice without either copy of the gene die before they’re born. So the key question is, does the reduction in mutant huntingtin that’s needed to treat the disease cause a reduction in wild-type huntingtin that’s dangerous?
At the moment we don’t know. Some researchers feel that only small reductions in mutant huntingtin will be enough to give cells the chance to recover, so we needn’t worry about harmful effects of lowering the wild-type protein slightly. Others believe that we need to develop treatments that will switch off the mutant protein only. That’s called allele-specific silencing – an allele is what we call each of the two copies of a gene.
Allele-specific silencing sounds very sensible – if we can target the mutant gene, why wouldn’t we? The downside is that to target just one copy of a gene, you have to look in each patient’s DNA for individual ‘spelling’ differences between the two alleles. Fortunately, such differences are common, but we’d still probably need several different drugs to be able to provide targeted treatment for as many patients as possible. Some patients don’t have any suitable spelling differences that could be targeted like this.
The debate about whether allele-specific silencing is necessary is ongoing, but the good news is that we won’t have to wait longer for an answer, because both techniques are being tested right now by different groups of researchers.
Challenge five: side-effects
Gene silencing treatments might still have side-effects, even if the possible problems with lowering wild-type huntingtin can be avoided.
One problem is so-called ‘off-target effects’, where the drug molecule sticks to, and interferes with, message molecules for genes other than huntingtin. That could produce any number of problems.
Another issue is that the brain’s immune system might end up fighting the ‘alien’ molecules that are being pumped into it, and that could make things worse.
We need to take these possible side effects seriously now, while we’re still at the stage of testing them in animals. Even quite mild unwanted effects could be bad news, especially if gene silencing ends up being used for many years to prevent symptoms in people who’ve had a positive test.
Scientists are working to produce the best molecules with the lowest risk of serious side-effects, and only the safest will be put forward for human trials.
When, when, when?
The excitement about gene silencing is justified, because many researchers feel it’s our best chance for producing successful HD treatments. There are clearly lots of issues to iron out, but with each passing year progress is being made, and everyone agrees that nothing has been found so far to suggest the technique won’t work in patients.
Right now a trial of ASO gene silencing is underway in patients with ALS (motor neuron disease). That’s really important because it’s testing not just the drug, but also the pump-and-tube delivery system to get the drug where it’s needed. Meanwhile, many researchers round the globe are figuring out which molecules will be best to test in HD patients.
We can’t say for sure when gene silencing treatments will be available for patients, but HDBuzz would be disappointed if we don’t see human HD gene silencing trials in the very near future.
Learn more
For more information about our disclosure policy see our FAQ…