COVID-19 update: what does it mean for HD families, how does it impact HD research, and how has it changed the way science works?

A collaborative team of scientists from Canada and Japan have identified a small molecule which can change the CAG-repeat length in different lab models of Huntington's disease. #HuntingtonsDisease #DrugDiscovery

HDBuzz reports from the annual Huntington’s disease therapeutics conference in Palm Springs

HDBuzz reports from the annual Huntington’s disease therapeutics conference in Palm Springs

HDBuzz reports from the annual Huntington’s disease therapeutics conference in Palm Springs

Scientists screen the ENTIRE genome to find new potential therapeutic targets for HD. This ambitious study provides a wealth of data for HD researchers

Researchers show that highly expanded CAGs in the HD gene can cause early developmental changes using 3D brain models called organoids. What’d they find?

Wave Life Sciences announces that its antisense drug WVE-120102 has lowered mutant huntingtin protein in cerebrospinal fluid, but investors seem disappointed. Rather confusing – what do we know for sure?

Researchers got surprisingly lucky when looking for drug molecules to pull mutant huntingtin protein into a cellular garbage disposal machine
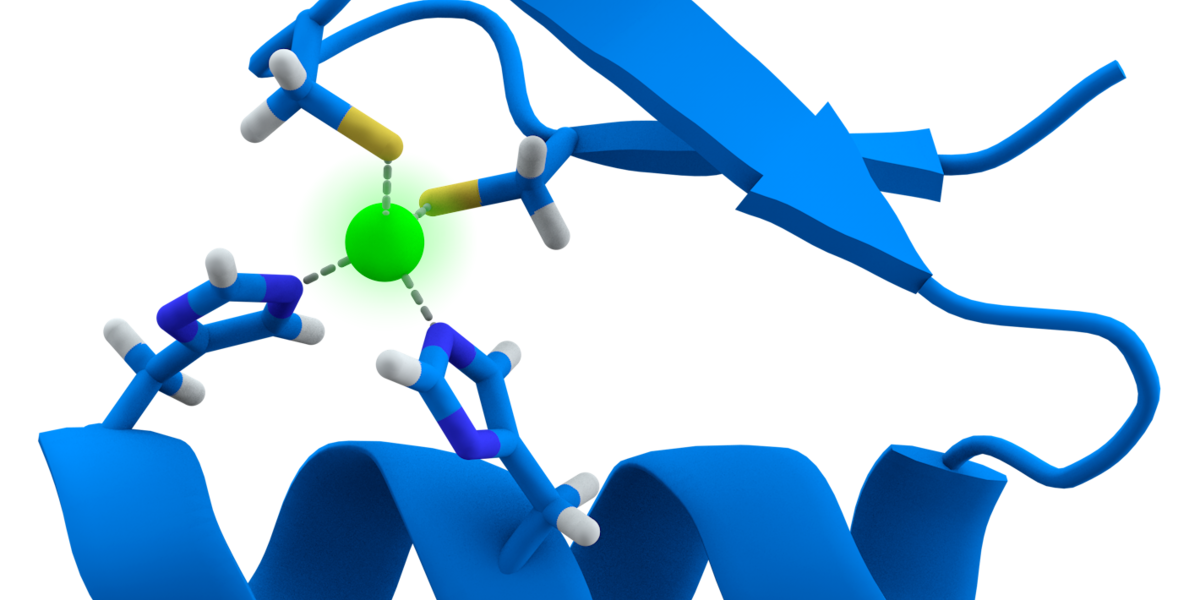
Exciting new Huntingtin Lowering work from @SangamoTx and @CHDIfoundation using "Zinc Fingers" to shut down expression of the mutant Huntingtin gene. More details on this exciting new technique here.
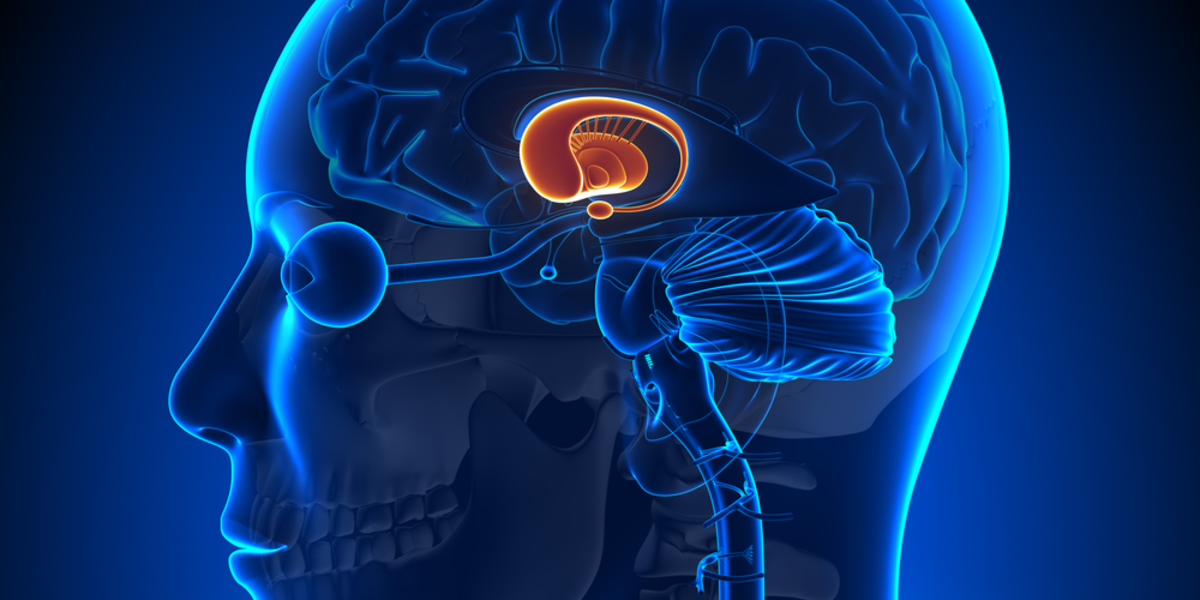
UniQure announces key details of its planned trial to assess the safety and ability of AMT-130 gene therapy to lower the problematic huntingtin protein using a ‘single-shot’ virus delivery system.