Mini brains grown in a dish shed light on Huntington’s disease and how we might treat it
Exciting new findings using 3D human lab-grown mini brains inform ongoing HTT-lowering trials and suggest that stem cell transplants for HD may improve cell-to-cell communication and reduce disease features.

Stem cells grown in 3D in a research lab can mimic some features of Huntington’s disease (HD). They also hold promise for transplantation studies to potentially add back cells that are lost in HD. But what would happen to those new cells? Would they get along with the cells still in the brain that have the HD gene? And what can this system teach us about ongoing clinical trials aimed at lowering the HD-causing message in only parts of the brain? Read on to find out!
The power of stem cells
Stem cells hold a certain mystique. They can either retain their “stemness”, remaining a stem cell, or to turn into something else altogether. Contained within each one is the ability to become almost any cell type in the human body. Scientists can coax them into becoming a heart cell, or a muscle cell, or even a brain cell, providing scientists with a powerful research tool that can be used to answer questions about people’s brains in health and disease.
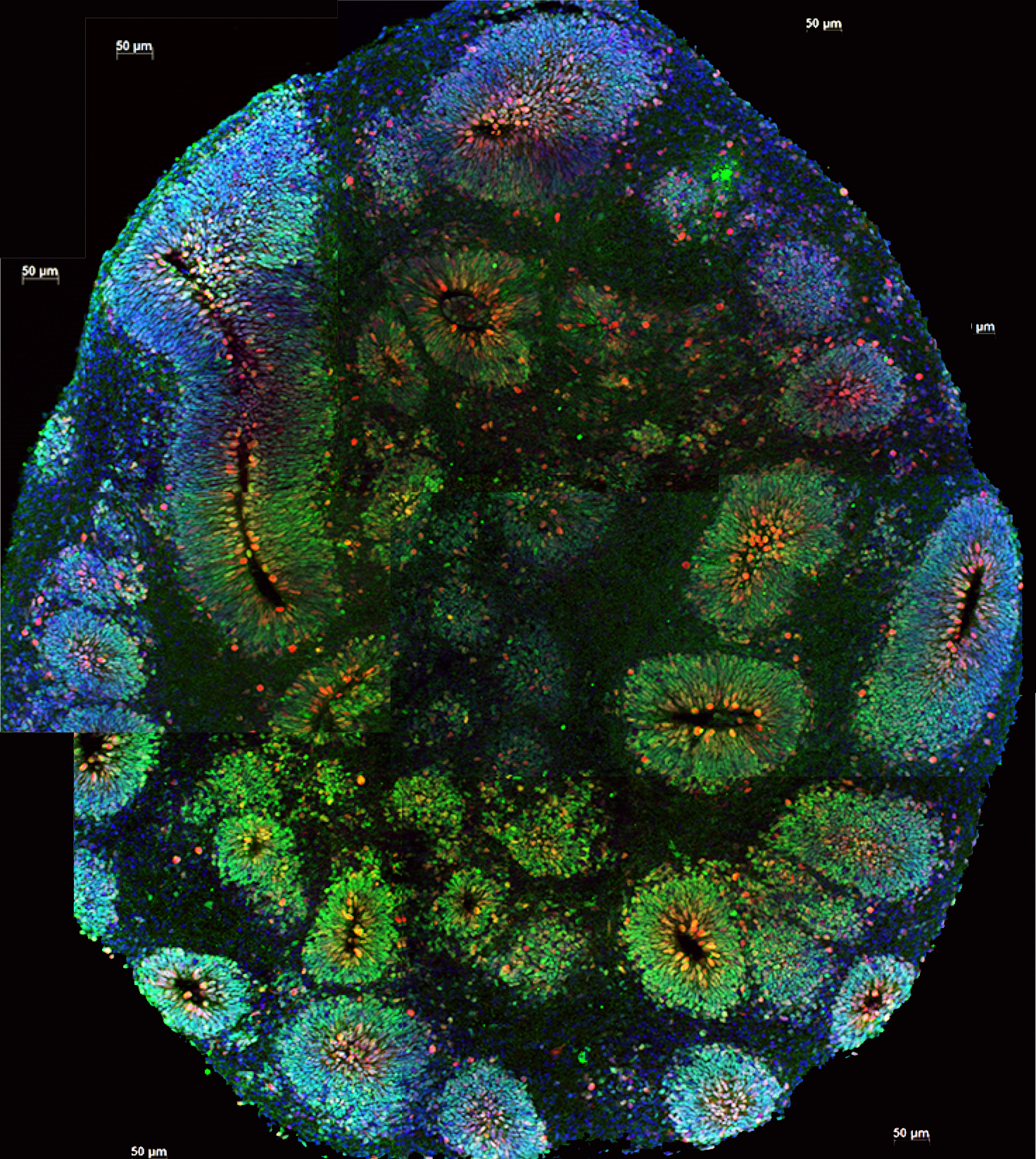
Image credit: Vaccarino Lab, Yale University
For brain diseases like Huntington’s disease (HD), there’s a second powerful potential application for stem cells – transplantation. As a neurodegenerative disease, HD causes the gradual loss of brain cells. This primarily happens in a central portion of the brain, called the striatum, and in the outer wrinkly bit of the brain, called the cortex.
Several groups of researchers are exploring approaches that would allow them to harness the power of stem cells to replace cells that are lost over the course of HD. We recently wrote about the work Dr. Leslie Thomspon is advancing for stem cell transplants from our coverage of the Hereditary Disease Foundation conference. But what would happen to the new cells? Would they adopt features of HD?
Dr. Elena Cattaneo and her team from the University of Milan, in Italy, recently published a study aiming to answer some of these questions. Elena’s lab are world leaders in using stem cells to research HD. In this new paper, they sought to better understand the effect that cells with the gene for HD have on cells without the HD gene. This might help inform future cell transplantation studies and trials aimed at lowering the disease-causing message since those drugs are unlikely to hit every cell in the brain equally.
Mini brain in a dish
Typically, when cells are used in lab experiments, they’re grown flat on the back of a dish. But if you’ve ever seen another person, you know that people aren’t 2D! So more sophisticated technologies allow researchers to grow cells in 3D.
The fancy term for these 3D cells is “organoids”, aka “mini brains”. We’ve previously written about these lab-grown brains and what researchers have learned from them. While mini brains can adopt some of the cellular features of a brain, such as connections between different cells, they don’t actually have the ability to transmit thoughts and feelings.
“While these mini brains look deceivingly unsophisticated on the outside (like a little whitish, pinkish snot to be honest!), they’re elegantly complex on the inside.”
While these mini brains look deceivingly unsophisticated on the outside (like a little whitish, pinkish snot to be honest!), they’re elegantly complex on the inside. The cells form intricate networks between brain cells that can be seen communicating with one another under the microscope. These mini brains give researchers a way of understanding in 3D how HD affects connections and communication between different cells.
Scientists know that in a human brain, HD reduces the ability of cells in the outer cortex to communicate with the inner striatum. This communication breakdown leads to a loss in those connections over time. When those connections go unused for extended periods of time, it can create an unhealthy environment for the brain cells, and they may eventually die.
A positive influence
Elena and her team see something similar in their mini brains that have the HD gene. At the molecular level, brain cells communicate across a very small gap called the synapse. This is where the tips of brain cells meet to send bubbles of information back and forth to one another. In HD, the number of bubbles is reduced over time. In this new paper, the team sees the same thing in HD mini brains – there is less communication at the synapse than in mini brains without the gene for HD.
A key experiment in the new paper from Elena’s lab asked what happens to cells in mini brains when cells without the gene for HD are combined with cells that have the gene for HD.
The team performed a very detailed analysis of the genetic messages contained in the mixed population mini brains, seeing that they more closely resemble the mini brains without the HD gene rather than the ones with the HD gene. This suggests that the cells without the HD gene have a positive influence on those with the HD gene. Good friends to have around!
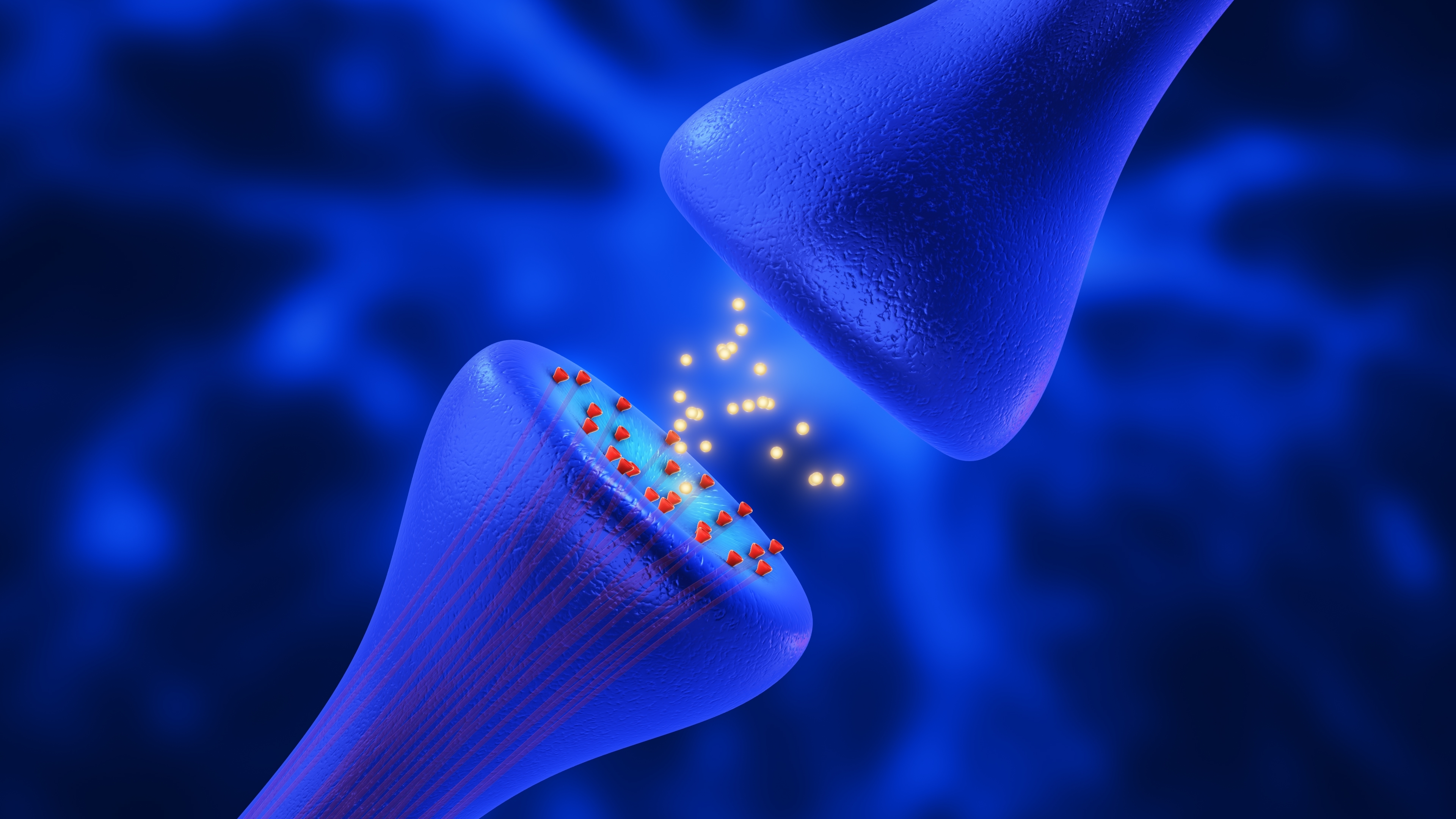
They also looked at the synapses in these mixed population mini brains. They found the communication being sent from the synapse was greatly improved! It more closely matched the mini brains without the gene for HD. This suggests the cells without HD might be helping the cells with HD to communicate better.
The team also identified some features that weren’t totally rescued by the presence of the cells without the gene for HD. In the mixed population mini brains there were still some changes at the genetic message level. Additionally, the number of cells that died in the mixed population mini brains wasn’t totally rescued. This suggests that while cells without HD help the mixed population mini brains, they can’t overcome every feature caused by the HD gene.
Informing ongoing and future trials
Overall, this type of research can help determine the therapeutic potential for using stem cells to slow progression and treat HD. It is also informative for ongoing trials that lower levels of the disease-causing genetic message.
While the goal for some of those trials is to lower the message by about 50%, that won’t occur in every cell in the brain. Because of that, those cells with reduced HD genetic message will exist in a mixed population with cells that have more of the HD genetic message. Data from studies like those highlighted here help researchers understand exactly what may happen at the molecular level when such mixed populations of cells with and without the gene for HD exist.
An important point the research team was able to tease out in this paper is that the cells without HD have a positive influence on the cells with the HD gene. But the opposite is not true. The cells that have the HD gene don’t seem to alter programs in the cells without HD. This is important for future transplantation studies because it suggests cells without HD that are added may have a positive effect, but the cells already in the brain with HD possibly won’t have a negative effect on the new cells. A win, win!
“This suggests that the cells without the HD gene have a positive influence on those with the HD gene. Good friends to have around! ”
Moving treatments forward
While stem cells and mini brains are super cool, there are some limitations to their use. Firstly, they don’t truly mimic what’s happening inside a human brain in a living person. Nothing in a lab dish can. This is why it’s important to study potential treatments in a functioning brain, like in a mouse, and eventually run clinical trials in people.
Additionally, the mini brains that contained cells with and without the gene for HD were mixed before they were made. Meaning the mixed population was there from “birth”. In the case of a person with HD, the cells or treatment would be added after the person had a fully formed brain.
Despite the caveats, this work represents a cool approach for better understanding how cells without the gene for HD may act if they were added to a brain with HD. It also sheds light on what may happen in a brain when some cells have the gene for HD while others have less of that message.
The human brain, both inside a lab dish and out, is incredibly complex, so knowing as much as possible about how HD affects cellular and molecular features will help move treatments forward.
Learn more
For more information about our disclosure policy see our FAQ…


