Tipping the balance; new insights into HD genetic modifiers
A new study from researchers at Thomas Jefferson University delves into the details of how genetic modifiers of Huntington’s disease work.

Genetic modifiers can influence when HD symptoms begin. Some of these genes encode for different types of molecular machines whose normal job is to repair our DNA when it is broken or damaged. A recently published study from scientists at Thomas Jefferson University uncovers details of how these molecular machines help repair damaged DNA structures that can occur in HD, revealing a complicated balancing act.
In this article, we explore what the scientists found, how this can help us understand how different modifiers work to alter the path of HD, and ways these new insights might guide development of new therapies.
Genetic modifiers of HD change the age at which symptoms appear
Every case of HD is caused by the same genetic change, the extension of a long stretch of the letters “CAG” in the Huntingtin gene. An intriguing mystery in HD research has been the fact that folks with the exact same CAG number can often start to get symptoms at very different ages.
To better understand why this is the case, in a number of studies now, scientists looked at DNA samples from thousands of people with HD and looked to see what small letter changes in their DNA code tallied with symptoms starting earlier or later in life.
The genes they identified in these studies are called genetic modifiers as they modify the course of HD, from what we might expect based on the CAG number alone. Interestingly, many of the genes identified in these modifier studies encode molecular machines (proteins) whose normal role in the cell is to repair DNA when it is broken or damaged.

Two such modifiers are FAN1 and MSH3, which are the focus of this research study. However, MSH3 doesn’t work on its own, it has to be together with another molecule called MSH2. One way to think about this is to consider how we make bread; yeast on its own is not enough to make the bread rise, it needs to be together with water and flour to be active and work properly. Similarly, MSH3 needs MSH2 to work, and the assembly they form together is called MutS Beta which is what Pluciennik and colleagues studied in their experiments.
DNA repair is a double-edged sword
The huntingtin gene contains a long string of “C-A-G” DNA letters repeating over and over. In people without HD this CAG number is usually less than 35, but in people with HD, the CAG number is more than 35.
Long strings of the CAG letters in DNA code can make strange shapes and structures with mismatches in the DNA helix, some of which are called extrusions. DNA damage repair machines recognise and work on these mismatches and extrusions, to try and restore them back to regular looking DNA strands. If cells fail to repair their DNA correctly, a number of bad things can happen, including the development of cancer.
Sometimes, these molecular machines are rather sloppy and can actually make things worse, adding in more CAGs into the huntingtin gene, a process called somatic expansion. In particular, MutS Beta has been shown to jump onto CAG extrusions and can make long CAG repeats even longer over time. On the other hand, FAN1 does a much better job of chopping out the damaged bits of DNA and ensuring the DNA code is faithfully maintained with no additional CAGs.
The battle of the molecular machines!
In this new study, Pluciennik and colleagues investigated how different molecular machines, FAN1 and MutS Beta, get recruited to these CAG extrusions and how they repair them.
First, the team showed that FAN1 can work on the CAG extrusions, but not on its own; other DNA repair proteins need to be present too and the chemical conditions have to be just right. One of the most important partners for FAN1 is a cool looking star-shaped protein called PCNA which clamps onto the DNA strand and helps other proteins, like FAN1, climb on too.
Next, the scientists showed that MutS Beta can push FAN1 off the DNA extrusions and stop it from working properly. Interestingly, the team found that the precise balance of MutS Beta and FAN1 was very important as to which molecular machine got to work on the extrusions. If there is more FAN1 than MutS Beta, the FAN1 wins and can get to work to start repairing damage on the DNA.
But what does this mean for HD research?
While understanding the precise minutia of how these molecular machines work may seem a million miles away from finding a cure for HD, the impact of this type of science can be very important for drug discovery.
The identification of genetic modifiers of HD gives scientists some of the best clues for how to make new medicines. These gene lists provide crucial insight about which proteins could be switched on or off, in the hope of delaying HD symptoms.
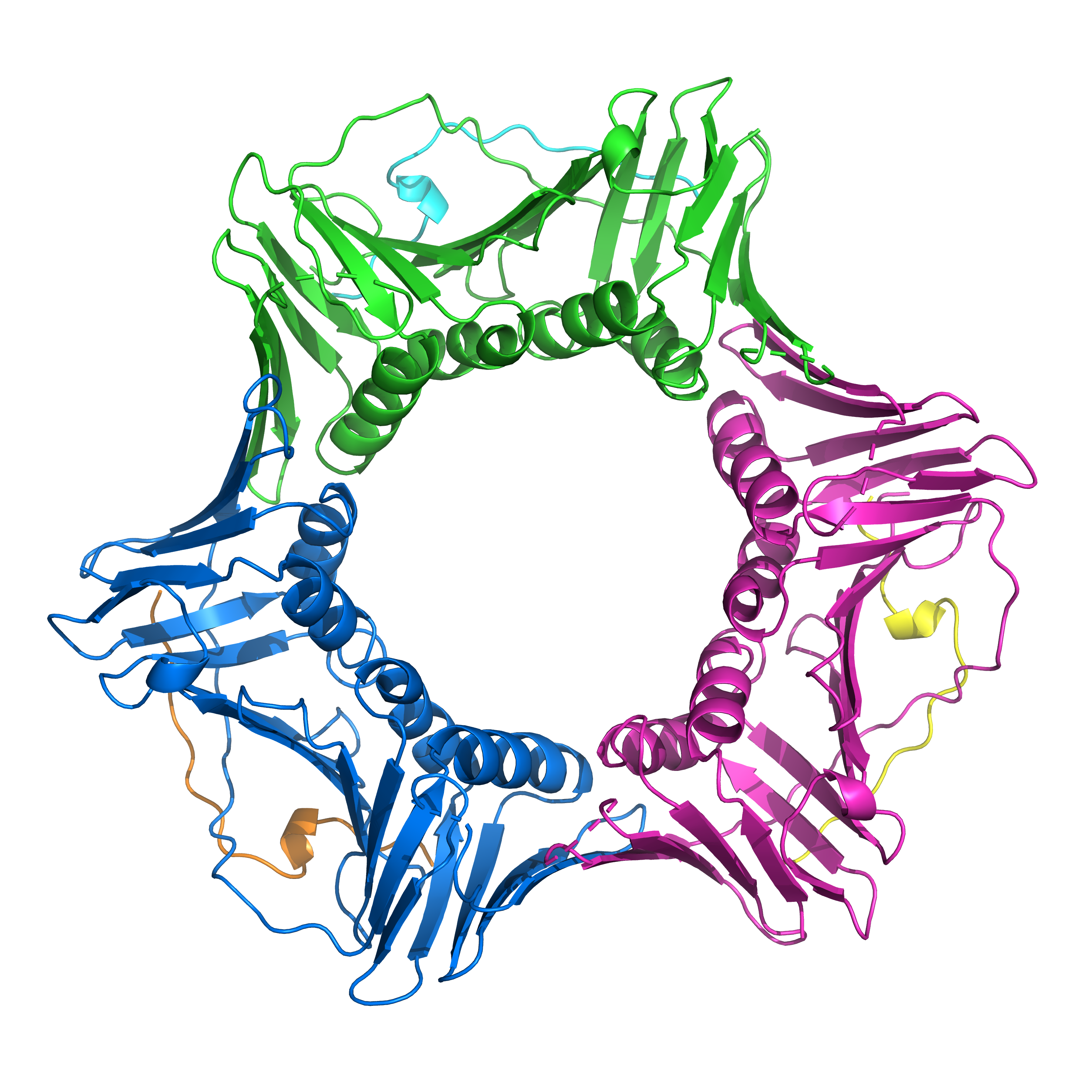
It’s because of thousands of HD patients and their families that donated DNA to research efforts that scientists were able to discover that both FAN1 and MutS Beta can influence the age of onset of HD. This new paper by Pluciennik and colleagues shines a light on some of the cool details of two of these modifiers, and the delicate balancing act between FAN1 and MutS Beta during repair of CAG extrusions.
Studies like this will in turn help drug hunters focused on these pathways to conduct better experiments as they attempt to refine and develop new drugs for HD.
Learn more
For more information about our disclosure policy see our FAQ…