
Huntington's disease therapeutics conference 2021 – Day 2
Catch up on all of the latest updates from day 2 of the 2021 CHDI Huntington’s disease therapeutics conference #HDTC2021




We are back with Day 2 of the virtual 2021 CHDI Therapeutics conference. This article summarises our live Twitter updates on the exciting science being presented, which you can continue to follow with the hashtag #HDTC2021. The morning session focussed on promising HD therapeutics that are in preclinical development and the afternoon session covered different biomarkers for Huntington’s disease.
What’s coming down the pipeline for new drugs to treat Huntington’s disease
In addition to all of the clinical trials which are currently underway for Huntington’s disease, there are lots of researchers still looking for and developing new and innovative ways to treat HD. This session highlighted some of those promising approaches.
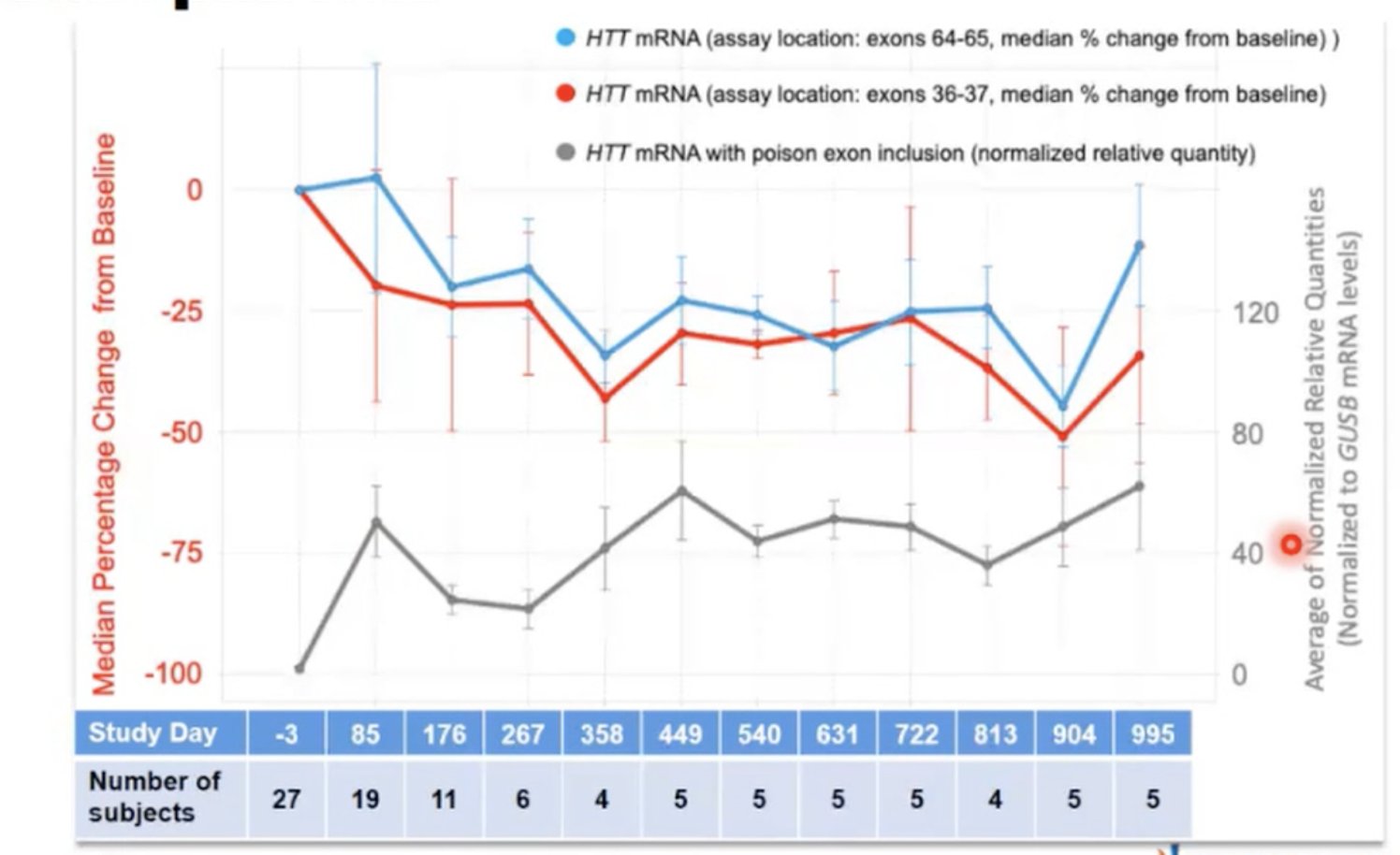
Image credit: Novartis
Fixing the trash system in our cells to get rid of toxic huntingtin protein clumps
The first speaker was Peter Hamley from Samsara Therapeutics, who spoke about small molecules to boost autophagy, a type of trash disposal system in cells. Brain cells can use autophagy to clean up debris, including toxic disease proteins like mutant huntingtin. Designing drugs to boost huntingtin removal is another approach to protecting brain cells. Autophagy is linked to aging in many different ways; enhancing autophagy with different tools can increase the lifespan of animals in the lab and might be used to treat age-related illnesses like Huntington’s disease.
Samsara is using a screening process to find molecules that boost autophagy, and then they test their “hits” to see if they can improve removal of toxic proteins. Using their screening platform, Samsara has identified a few interesting compounds that can boost autophagy in cells and mice. These compounds seem to perform better than previously discovered drugs and chemicals that work to enhance autophagy, and they look promising in other models of neurodegeneration, like Alzheimer’s. Recently applying this work to HD, Samsara went on to show that their compounds are protective in HD cells and flies. Hamley shared that they hope to have identified and developed their best molecule in the near future, with the view to nominate a drug candidate for further testing in 2022.
Small molecules that lower mutant huntingtin
Next up was Beth Hoffman from Origami Therapeutics, an (aptly named) company that is focused on protein folding and misfolding. Because of the extra CAG repeats, expanded huntingtin folds into a different shape which could be why it’s more likely to get chopped up into toxic bits. Origami Therapeutics is developing small molecules to encourage removal of these pieces of harmful huntingtin. They are hoping to find small molecules which either trigger cells to get rid of the toxic huntingtin protein, or to correct its shape, stabilising the 3D structure to make it less harmful.
Origami’s screening process measures buildup of the expanded huntingtin protein as well as damaging effects which they believe are caused by toxic clumps of protein. They aim to identify small molecules which correct these problems, then determine the best dose level and timing for delivering it to cells. Some of the compounds they have identified can reduce harmful huntingtin in cells, while others are believed to stabilize its structure to make it less harmful. Overall, Origami’s strategy is to determine which compounds are most helpful, and then work backwards to understand exactly how they act on the huntingtin protein.
Replacing sick support cells in the HD brain
The next speaker was Steve Goldman, an academic researcher who is exploring strategies to treat HD by replacing the brain’s support cells (glia) with healthy ones that don’t carry the HD mutation. We often talk about loss of neurons in HD, but other supportive brain cells are also affected.
Goldman’s research is investigating whether loss of glia could also be driving some of the symptoms of HD. He and his team take glial cells that make the toxic huntingtin protein, implant them into mice, and then run different experiments to see how this affects the mice. The develop a variety of symptoms and brain abnormalities compared to control mice.
He is also studying human cells grown in a dish, in order to explore the differences between glia that express the HD gene and those that do not. Glia with the HD mutation show many changes in the genes they have switched on or off, and brain cells surrounded by HD glia don’t work as well. One specific type of glia, called astrocytes, show changes in their shape, development, and health when the HD mutation is present.
Goldman’s research team was interested to find out whether implanting healthy glial cells into a mouse with HD could improve the symptoms of the HD mouse. In their experiments, HD mice with the healthy glial cell implants had somewhat improved behavioral symptoms and longer lives. The lab’s long term goal is to be able to move towards testing glial transplants in the human brain, and Goldman is working on planning a Phase I/II clinical trial. Such a trial would require special medical-grade glia, a bit different from the kind that most researchers study, and Goldman has been working with these too. There’s some work to do before a human study can come to fruition and it’s not yet clear what that timeline would look like, but it’s an interesting strategy.
“In addition to all of the clinical trials which are currently underway for Huntington’s disease, there are lots of researchers still looking for and developing new and innovative ways to treat HD”
From spinal muscular atrophy to HD therapeutics
This session’s final speaker was Rajeev Sivasankaran from Novartis. He spoke about branaplam, a drug which can be taken by mouth to lower levels of the huntingtin protein. You can also check out our previous HDBuzz article about branaplam and HD
Novartis’ interest in HD began in another neurodegenerative disease, spinal muscular atrophy (SMA), which causes severe muscle weakness in children. Like HD, SMA is inherited, but unlike HD, it’s caused by a missing protein rather than the presence of a toxic protein. To treat SMA, Novartis developed branaplam, a drug that restores the missing protein by a trick called splice modulation.
Branaplam allows cells to switch on another gene whose protein can replace the missing function. That gene is normally dormant, but branaplam restores its activity. Branaplam was tested in human patients and overall performed well. It’s still being studied but so far looks good for SMA kids.
What has this to do with HD? Branaplam works by altering splicing of mRNA, the working copy or “recipe” of the gene that is generated on the way to making a protein. Turns out, the way the drug acts to activate the SMA-rescue gene could also make it active on the recipe for huntingtin protein. By a happy coincidence, the Huntingtin gene contains the same sequence, in a place where branaplam’s effect could reduce production of the protein. It does this by forcing the inclusion of a sequence that would normally be deleted – a sequence that says “stop making this protein.” The effect of branaplam is a bit like adding a missing chapter to a book that says “stop reading this book.”
But does this actually work in living creatures? Yes, in mice with a human mutant HD gene, anyway. Sivasankaran presented data showing that the level of huntingtin goes down at 3 weeks after treatment with branaplam, then comes up again.
And of course, branaplam has already been tested in human kids with SMA! In the trial, the huntingtin levels of the branaplam-treated kids fell by about a third, then stayed low. Novartis is in the advanced stages of planning a human trial of branaplam in Huntington’s disease, but no details of that trial were revealed today. During the Q&A, Sivasankaram hinted that more information about the trial program will be revealed “in the coming weeks” and “in the summer.”
Biomarkers For Huntingtin-lowering Programs
In the afternoon session today we heard from researchers working on biomarkers of Huntington’s disease. Biomarkers are different ways that scientists can carefully track the progression of HD and whether huntingtin-lowering treatments are working.
Brain imaging to detect levels of mutant huntingtin
Daniele Bertoglio kicked things off by describing his research which can help measure harmful huntingtin using non-invasive brain imaging. His team is developing sophisticated imaging tools in hopes of being able to detect huntingtin protein levels in the brains of HD patients.

Currently, we can indirectly measure levels of huntingtin in the brain by looking in the cerebrospinal fluid (CSF), which bathes the brain – we covered this previously here. Bertoglio’s team is working on a strategy that uses chemical tracers that stick to the huntingtin protein in the brain. They’re mildly radioactive, and so can be detected with a machine that does something called positron emission tomography (PET) scanning. PET scanners are widely used in medical clinics around the world, though they’ve never been used to measure huntingtin levels, because each new application of a PET scanner requires a new chemical tag that can be injected into people being imaged.
Using mice treated with drugs that reduce huntingtin protein levels, Bertoglio’s team has been testing a new huntingtin tracer. Mice with lower huntingtin levels have lower signal on the PET scanner, as they’d hoped. The team has also studied mice in which huntingtin levels are lowered in a small, limited, area, as well as more widely, using different techniques. The Huntingtin PET tracer works in both cases, suggesting it could be used to detect both types of lowering.
Bertoglio also mentioned during the Q&A that his team has tested this new tracer in donated brain tissue from HD patients, where they can see it binding. This is exciting, as it suggests the tracer could work in the brains of living HD patients.
Testing huntingtin tracers in humans
Andrew Wood spoke next about some more imaging ligands which can help us measure how toxic huntingtin protein builds up in the brain. His group is conducting the iMagemHTT study, which is a clinical trial of the huntingtin tracer discussed in the previous talk. They are doing simultaneous blood sampling and PET/MRI imaging to understand how it tracks huntingtin and gets processed by the body.
Wood has carefully studied different doses of two huntingtin tracers in humans and how long they last in the body and brain. They have moved forward with the one that seems best and safest. Wood presented preliminary data from three participants in a Phase I study of the tracer, including data about safety and how it breaks down over time. So far all is looking good and they will continue to add participants to the study.
The trial will progress over the course of the next year, and participants with and without HD will receive the tracer and undergo imaging and bloodwork. If successful it could potentially be used in future trials to measure huntingtin-lowering in the brain. In the background, Wood’s group in collaboration with CHDI are also working on testing the properties of a few other candidate tracers, as backup.
Because huntingtin levels in spinal fluid might not reflect huntingtin levels in the brain, a tracer like this has exciting potential as a useful tool for tracking HD progression or effectiveness of therapies. Wood hopes that the top candidate will make it to the clinic by sometime in 2022.
Assessing HD biomarkers in a non-human primate model of HD
Jodi McBride talked next about her research studying biomarkers in non-human primate models of Huntington’s disease. Most HD-related studies in primates work with healthy monkeys to understand things like how drugs can be delivered to a large brain. McBride has developed a novel model of HD in which a virus delivers the HD gene to the brain, leading to buildup of mutant huntingtin in monkeys. These monkeys have problems with working memory and movement tasks that are somewhat similar to HD symptoms, as well as brain changes similar to human HD.
McBride’s team are continuing to explore imaging and visualisation methods, including those described by Bertoglio and Wood, to confirm that these HD monkeys will be a useful model for studying HD biomarkers.
“Biomarkers are different ways that scientists can carefully track the progression of HD and whether huntingtin-lowering treatments are working”
Innovations in brain imaging to track HD progression and define new biomarkers
Derek Jones spoke next about new MRI technologies which allow non-invasive imaging of the brain that might be better deployed to track progression of HD and different biomarkers of disease. Jones recapped the promise that imaging methods hold for understanding how neurodegenerative diseases work and how we might treat them. Even with very early MRI studies, changes to HD brain structure were observed and gave us clues to how HD was affecting patients.
However, Jones highlights that interpreting imaging data and making good measurements is very challenging. Lots of different changes in the brain might all look the same when we look at the brain MRI data so teasing apart what’s what is very important for HD scientists. Much bigger and more powerful MRI machines will help to spot these differences more easily and these are becoming increasingly mainstream in hospitals. These new machines also let us see parts of the brain which were previously difficult to image but are important to disease – like the striatum which is an important region of the brain in HD.
Jones’s lab is also designing new ways to run experiments for brain imaging so that doctors and clinicians might get the most information possible from MRI studies, seeing every possible detail of the brain structure. These new methods involve lots of complex mathematics! We’re lucky to have these smart folks working on HD research. Using these cutting-edge techniques, Jones’s lab are able to see very subtle differences in brain structure in people with HD who have yet to exhibit symptoms.
Jones then gave a shout-out to HDClarity which is standardising the collection of CSF samples from HD patients across the globe. He is applying the same principles to imaging technology in a project called ImageClarity.
Hunting for new biomarkers in CSF for HD
The final talk of the day is from Niels Skotte, who told us about his research tracking the levels of different proteins in the spinal fluid and blood of people with Huntington’s disease. Skotte is looking for novel biomarkers which might track with HD progression in patients.
Using just tiny amounts of blood or spinal fluid, Skotte and colleagues are able to identify which proteins they find in different samples for people with HD at very different stages of the disease. Skotte and colleagues find significant protein changes in both CSF and blood samples when they compare control samples, HD patients with symptoms, and pre-symptomatic HD gene carriers.
Skotte sees good correlation of a protein called NfL, which was previously identified; levels of NfL increase as disease progresses in people with HD. This is already used as a biomarker in clinical trials for HD. Another protein Skotte finds is called PENK, and it decreases over time as disease progresses in people with HD. Skotte and colleagues are continuing to evaluate these new potential biomarkers to validate how they track with disease.
Until next time…
And that wraps up the talks for today folks! We will be back for the final day of presentations tomorrow.
For more information about our disclosure policy see our FAQ…


