
Sleep, cilia and HD
New studies shed some light on the function of sleep in animals, with interesting implications for HD research
Studies have shown that HD patients tend to get less efficient sleep, fewer hours of sleep, and wake up more times during the night. However, sleep in Huntington’s is under-researched because historically scientists have investigated HD as a disease of movement impairment, and sleep problems don’t seem to have anything to do with movement impairment.
Sleep – what is it good for?
The picture is a lot more complex now. Huntington’s disease clearly involves more of the brain than just structures involved with movement. It now appears that sleep — that evolutionarily dubious activity that takes up one third of our lives — may come into play in important ways.
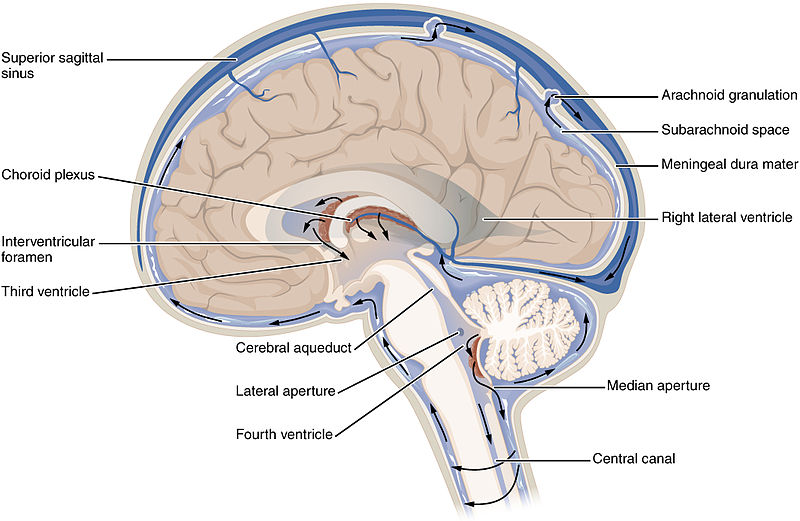
Image credit: Wikicommons
It’s well known that sleep is indispensable to health and well-being, that with minor sleep deprivation, mood, reasoning ability, and learning suffer; with moderate sleep deprivation, our immune systems are less effective and even our hormones go out of whack. In HD, sleep deprivation may have an even more potent effect.
Sleep may be protective in HD
Some symptoms of HD, like thinking impairment and clumsiness, look a bit like symptoms of chronic sleep deprivation. Scientists now believe that sleep deprivation commonly occurs in HD, hidden among other symptoms, and possibly acting in the progression of the disease.
So far there have been no systematic studies to determine whether sleep deprivation is the cause of any HD symptoms. This is an exciting area of study because if dysfunctional sleep is indeed the cause of some HD symptoms, it becomes a strong contender to be the focus of a treatment.
The treatment of sleep disorders in HD patients has also not yet been systematically studied, but there is actually some evidence that imposing a regular sleep schedule is “protective” in mouse models of HD.
In one study, mice carrying the HD mutation were injected with sleep-inducing drugs every night to force them to sleep. In a (perhaps surprisingly) standard test of learning and memory, researchers placed the mice into water-filled tanks with a light signifying the location of a submerged platform. Since mice would much rather stand on the platform than swim, the researchers could observe how well the mice learned and remembered that “light means platform” by the direction they initially swam on repeated trials.
Scientists believe that this type of learning and memory is tied to brain structures especially affected in HD patients. The sleep-regulated mice performed better on this task, suggesting preservation of these brain structures, or at least their function.
“A new study by Dr. Nedergaard of the University of Rochester, New York, suggests that the value of sleep may lie in helping to clean out the brain. While not focused specifically on HD, the study raises interesting questions about the role sleep plays in diseases like HD.”
This study is obviously very far removed from being a test of viable treatments in people — it certainly doesn’t tell us that chemically induced sleep would improve the overall health of HD patients. What it does provide is a kernel of evidence that dysfunctional sleep is harmful in the progression of HD.
Sleep hormones help HD mice
One way the body naturally regulates sleep is with a “hormone,” or chemical messenger, called melatonin. Release of melatonin by the brain signals that it’s time to sleep and consequently we feel drowsy.
HD patients have been found to produce less melatonin at night, and in fact this may contribute to the dysfunctional sleep sometimes experienced in HD. To see how melatonin levels might be affecting HD patients, researchers injected Huntington mutation mice with extra melatonin every day. These mice lived longer and showed less brain deterioration than HD mice given a dummy injection.
Is this “protective” effect of melatonin related to its ability to regulate sleep? This is one possible explanation, although a protective effect of melatonin was observed in a plate of HD cells as well, which do not technically sleep. For a more in-depth discussion of this melatonin research in HD, you can read this http://en.hdbuzz.net/057 article on HDBuzz.
We know that in HD, clumps or “aggregates” of a specific protein called “Huntingtin” build up inside brain cells, where they disrupt important cellular processes. For cells, especially the long-lived cells of the brain, getting rid of old and damaged materials is vital, and it seems like this job is not getting done correctly in HD.
How does the brain dispose of garbage?
A new study by Dr. Nedergaard of the University of Rochester, New York, suggests that the value of sleep may lie in helping to clean out the brain. While not focused specifically on HD, the study raises interesting questions about the role sleep plays in diseases like HD.
One way cells get rid of the junk they can’t recycle is to expel it into the fluid between cells, called the “interstitial fluid”. Part of daily bodily maintenance involves clearing out this space, and for most of the body this is taken care of by the lymphatic system — a complex system which acts as both a gutter and a filter for interstitial fluid and is tied to the immune system. A fluid called lymph, which is essentially blood plasma, soaks into body tissues and flushes out waste.
The brain does not have access to the lymphatic system but it still needs to clean between its cells — perhaps more than the rest of the body — so it uses a similar system. The fluid that bathes the brain, called cerebrospinal fluid or CSF for short, does the job of the lymph, flushing out the soiled interstitial fluid.
Dr. Nedergaard’s team wanted to know how well the brain’s natural washing machine could remove some problem proteins and other cellular waste, so they injected some of these substances into the interstitial fluid of mice brains.
When they checked how much of the different substances remained, they were pleased to find that the brain had done a pretty good job of flushing them out. One protein that was surprisingly well cleared out was amyloid-beta, also known as Abeta. Abeta is the main constituent of the big clumps of amyloid protein found between sick neurons in the brains of Alzheimer’s disease patients.
The root cause of Alzheimer’s is still unknown but scientists have long suspected that build-up of Abeta and the resulting clumps between cells called “plaques” may be responsible for poor communication between neurons and the large amount of neuron death observed as the disease progresses. In this way Alzheimer’s disease is quite similar to Huntington’s: both involve clumping of protein that is toxic to surrounding neurons.
The Abeta found dissolved in the interstitial fluid is not the same Abeta bound up in plaques, but there’s some evidence that the amounts of the two are related.
Does this mean that a more efficient job of cleaning up the Abeta dissolved in the interstitial fluid could reduce the amyloid buildup? This remains to be tested. Either way, this is certainly a valuable finding for Alzheimer’s disease researchers, though it only loosely concerns HD.
Taking out the trash each night
Nedergaard and his colleagues’ next query would lead them to a more universally applicable question: that of the function of sleep. They knew from earlier studies that more Abeta is found in the interstitial fluid of awake than in sleeping mice and humans. So they wondered whether Abeta is washed away better during sleep or if just a smaller amount is created.
“This groundbreaking work by Dr. Nedergaard raises a lot of questions. Might the messed up sleep in Alzheimer’s disease be affecting the clearing of protein buildup and contributing to the disease? Might dysfunctional sleep be affecting protein buildup in HD as well? We don’t know yet, but you can bet that scientists are working to find out.”
To test this question, they trained mice to fall asleep while hooked up to testing equipment and repeated their earlier procedure of injecting waste substances into their interstitial fluid. In sleeping mice, the clearing of waste was much more efficient and, remarkably, Abeta was flushed out twice as well as when mice were awake.
What could explain the dramatic effect sleep had on the efficiency of brain cleaning?
One simple explanation is that during sleep some brain cells shrink to increase the area between cells. If this were the case, the river of fluid flowing through brain tissue would be wider, carrying away more trash. A test confirmed that the interstitial space was indeed much larger in the brains of sleeping mice.
This groundbreaking work by Dr. Nedergaard raises a lot of questions. Might the messed up sleep in Alzheimer’s disease be affecting the clearing of protein buildup and contributing to the disease? Might dysfunctional sleep be affecting protein buildup in HD as well? We don’t know yet, but you can bet that scientists are working to find out.
Sleep to the rescue?
These new results may be able to give new context to older findings in HD research. As HDBuzz has previously reported, work from several groups of HD scientists has shown that “cilia” don’t work correctly in HD brains.

Cilia are the microscopic cellular paddles that control the flow of CSF in the brain by beating in synchrony, pushing CSF throughout the brain. In HD the cilia of brain cells are poor paddlers and consequently CSF flow is reduced.
This new study by Nedergaard gives us added perspective on how dysfunctional cilia could be contributing to HD. The question becomes: is there a connection between altered sleep in HD patients and the buildup of harmful clumps of protein in their brains? Furthermore, do these problems have anything to do with the altered function of cilia in HD patient brains?
It’s important to be aware of the limitations of what we can take directly from these studies on sleep and ABeta. For one thing, they were conducted on mice and it’s quite possible that mice brains act differently than human brains during sleep. Also, none of Dr. Nedergaard’s studies were directed at HD, which involves the buildup of a specific protein inside of a cell, not outside cells. Thus, how much this information affects what we know about HD definitely remains to be seen.
With these caveats in mind, it’s totally worth getting excited about the many new scientific questions raised by this work. New dotted lines have formed — they’re just waiting to be filled in or erased.
Learn more
For more information about our disclosure policy see our FAQ…


