
Major Roche-Isis deal boosts Huntington's disease gene silencing
'ASO' gene silencing drugs for HD boosted by major deal between Isis Pharmaceuticals and Roche

Isis Pharmaceuticals and Roche have announced a multi-million dollar deal to support the development of ‘gene silencing’ drugs to human trials. This is big news that secures the future of these exciting drugs for Huntington’s disease.
If you ask a hundred Huntington’s disease researchers what the most promising experimental approach to preventing and treating Huntington’s disease is, nearly all would say ‘gene silencing’, also known as ‘Huntingtin lowering’ treatments. Now, a major deal between Isis Pharmaceuticals and drug giant Roche promises to support the development of one kind of gene silencing drug, called ASOs, taking them through to clinical trials in patients as quickly and efficiently as possible.
What’s gene silencing?
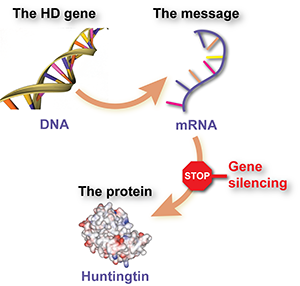
The cause of Huntington’s disease is the protein huntingtin, which is made throughout the body. Huntingtin is useful, but in HD, an abnormal form of the protein called mutant huntingtin causes damage, kills neurons and eventually produces the symptoms of the disease.
The instruction set for making the huntingtin protein – the huntingtin gene – is stored in every cell and is made of DNA. To make a protein, the cell first manufactures a working copy of DNA, from a related molecule called RNA. That ‘message molecule’ is then read many times by the protein-making equipment of the cell, which churns out lots of copies of the protein.
This RNA message molecule is the target of gene silencing drugs. The drugs are made from chemicals similar to RNA, and are designed to stick to the huntingtin message molecule but not to other message molecules. Once stuck, the drug tells the cell’s own machinery to dispose of the message molecule, so the protein isn’t made. That’s why gene silencing is also called huntingtin lowering.
There are different options for exactly what the drug molecules are made from, and several teams across the world are developing and testing different approaches.
So far, we’ve seen gene silencing drugs tested in several different animal models of Huntington’s disease, successfully delaying symptom onset or even reversing symptoms. These groups of researchers are now racing to hone their drugs and begin human trials.
Isis and ASO drugs
Anti-sense oligonucleotides or ASOs are one type of gene silencing drug, made from a DNA-like chemical. Isis Pharmaceuticals is the main driver developing ASO drugs for Huntington’s disease.
The main advantage of ASO drugs is that they naturally spread quite well through the brain when injected into the spinal fluid. In contrast, other gene silencing drugs with names like RNA interference, siRNA or shRNA, need to be injected directly into the substance of the brain and don’t spread very far without help.
“Beyond the money, the deal also gives Isis access to the significant resources and technologies of Roche.”
Last year, Isis announced successful safety trials of an ASO huntingtin gene silencing drug in primates, a crucial step on the road to getting a drug approved for human trials. Right now, Isis is at the stage of honing its drugs and deciding which one is best to take forward.
A pretty big deal
Developing ‘designer’ drugs is hard, and expensive, and testing drugs in human patients is the most expensive part. A fairly small company Isis would be unable to afford the huge cost of further development on its own, even with the support of existing partners like the CHDI Foundation. That’s why the newly announced deal with Roche is big news.
Essentially, Roche has committed to paying Isis $30 million for the development of its Huntington’s disease drugs and the first ‘phase 1’ clinical trial in patients. If that goes well, Roche will pay up to $362 million to further support the development and licensing of the drug.
Beyond the money, the deal also gives Isis access to the significant resources and technologies of Roche. One exciting future possibility is Roche’s brain shuttle technology, which aims to get the drugs into the brain without having to inject them into the spinal fluid.
Turning off one or both?
Every person has two copies of the huntingtin gene, one inherited from each parent. In most cases, Huntington’s disease is caused by just one faulty copy of the gene. Meanwhile, the normal copy of the gene produces a protein that does useful stuff and doesn’t cause harm.
Isis’s drugs that are closest to human trials target the message molecules from both copies of the gene – mutant and normal. So far, the early indications are that this approach is successful without causing harm. In part, that’s because neither copy of the gene is ‘silenced’ entirely.

But Isis is also working on drugs that only silence the mutant copy of the gene, reducing the potential for side effects. This is called allele-specific silencing. That’s a surprisingly difficult thing to do, because the place where the mutant gene is different isn’t necessarily the best place for a silencing drug to stick. So the drug-hunters have to look for other small differences between the two copies.
We don’t yet know what approach to gene silencing will be best, so it’s good to know that the deal will support both options.
Timelines
While it’s great to hear that a big pharmaceutical company like Roche is willing to commit such large sums of money to a Huntington’s disease drug, the big question for HD families is when clinical trials will begin in patients.
Both companies are understandably reluctant to commit to a specific deadline. In a conference call, Stanley Crooke of Isis said that “‘A little while’ is as precise an answer as I’m comfortable giving today”. Safety is paramount: it’s essential to get the drugs as good as possible and test them thoroughly before taking the risk of giving them to a human.
But it’s clear both Roche and Isis want to move forward as quickly as possible, and thanks to this huge deal, they now have the combined resources to make this happen.
Learn more
Sources & References
For more information about our disclosure policy see our FAQ…


