
Advances on many fronts in the battle against the protein that causes Huntington's disease
This fall sees exciting announcements from a number of companies focused on novel Huntingtin Lowering technologies, including Wave, PTC and Voyager

We’ve had a run of exciting updates from Ionis and Roche/Genentech about their program to test a drug that lowers production of the huntingtin protein – but they’re no longer the only game in town. Recently several other players – including Wave Life Sciences, PTC Therapeutics, and Voyager Therapeutics, have made big announcements about their own huntingtin-lowering programs. There’s a lot happening, and HDBuzz is here to help untangle all these approaches.
Huntingtin lowering
The goal, for all these approaches, is to lower the amount of huntingtin protein in brain cells. The huntingtin protein is the little machine made by cells as they follow the blueprint found in the HD gene. It’s the mutant huntingtin protein, not the mutation in the DNA, that most researchers believe causes the brain cell dysfunction that causes HD.

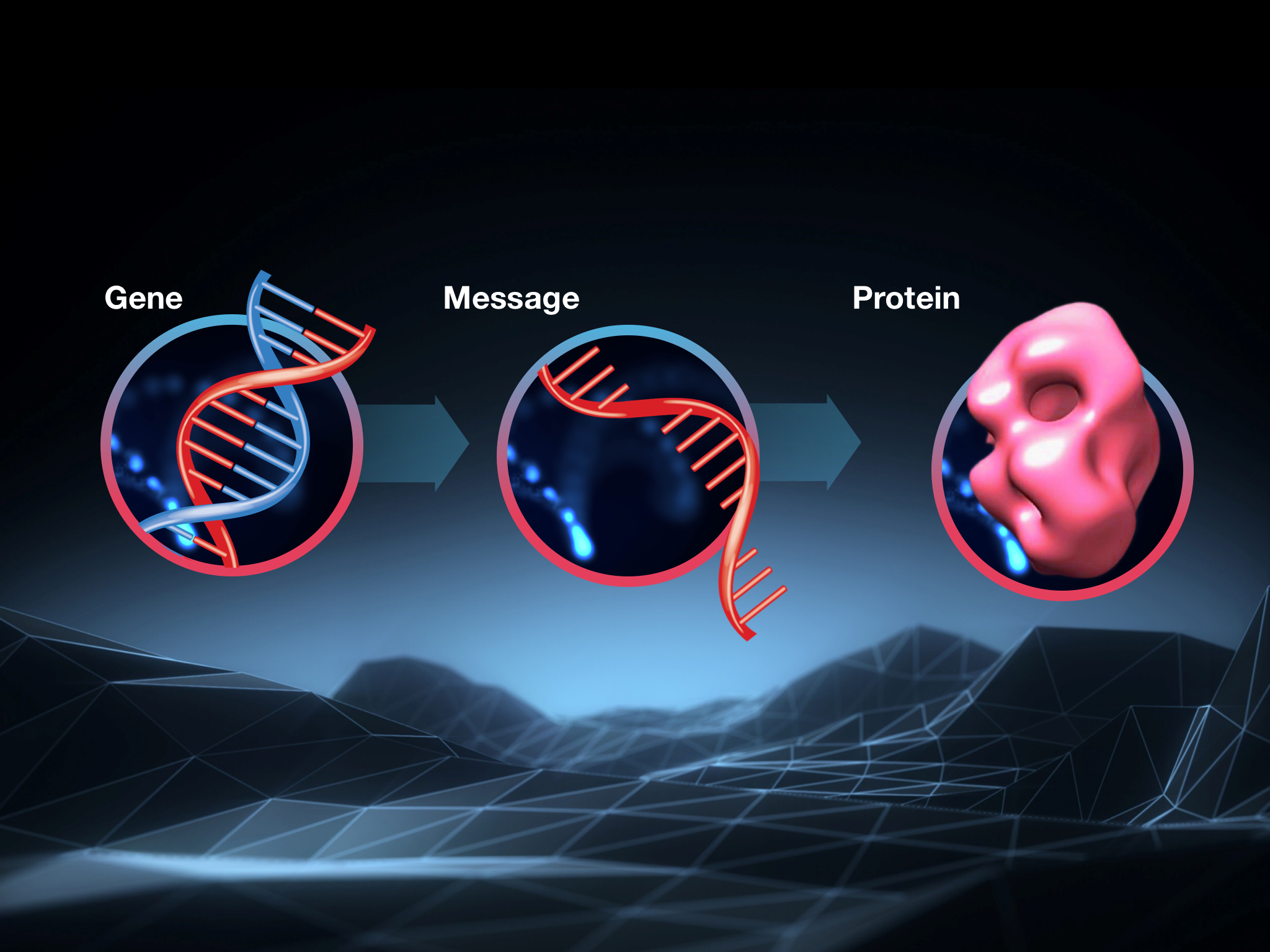
One quick reminder about how this process works is in order before we dive in. Cells follow instructions found in DNA, but they don’t use it directly to make a protein: they copy the instructions found in DNA into a sort of scratch copy of the genetic information, made from a closely related chemical called RNA. Scientists call this scratch copy messenger RNA or mRNA for short.
So the instructions in the HD gene, in our DNA, are copied into messenger RNA, which is then interpreted by the cell to build the huntingtin protein. A bit confusing, but it works!
If you only remember one thing, remember this: a break in the chain anywhere will stop cells from making the huntingtin protein. In animal studies, lowering huntingtin protein led to big improvements in HD-like symptoms.
This approach to HD therapy is called huntingtin lowering and it’s behind the Ionis/Roche program and forthcoming large phase 3 trial. Recently though, at least three other companies have announced exciting advances in various huntingtin-lowering approaches.
Wave’s targeted approach
“Wave’s approach targets tiny genetic differences between the healthy and mutant HD genes, outside the disease-causing CAG stretch. These minor spelling differences are part of normal human genetic variation, and appear to have no impact on HD symptoms. But the tiny spelling differences provide a target for an ASO that can discriminate between the normal HD message and the mutant one.”
First up is not one, but two trials from Wave Life Sciences. We’ve written about Wave’s approach here. Like the Ionis/Roche/Genentech drug currently being tested in HD patients, Wave’s technology is based on antisense oligonucleotides (ASOs) – small, heavily modified bits of DNA that enter cells, find specific messenger RNAs and destroy them.
As in the Ionis/Roche trial, Wave is targeting the huntingtin message for destruction in hopes of improving HD symptoms. But Wave is taking a slightly different approach. Remember that virtually every HD patient has one mutant HD gene and one normal copy. The Ionis/Roche drug targets both copies, ultimately reducing levels of both the normal and the mutant Huntingtin protein in the brain.
Wave’s approach targets tiny genetic differences between the healthy and mutant HD genes, outside the disease-causing CAG stretch. These minor spelling differences are part of normal human genetic variation, and appear to have no impact on HD symptoms. But the tiny spelling differences provide a target for an ASO that can discriminate between the normal HD message and the mutant one.
In a perfect world, targeting only the mutant HD gene is obviously better. The normal HD gene has a number of important roles in cells, not all of which we understand perfectly. If it were just as easy to remove only the mutant copy, that’s what we should do.
However, there’s always a tradeoff. For huntingtin lowering, the tradeoff is that not every HD patient is eligible for the ASOs being developed by Wave. For their approach to work, a person has to have inherited the HD mutation and also one of the genetic spelling differences that the drugs home in on.
Wave has conducted studies in HD clinics, and shown that up to about two thirds of HD patients may be suitable for treatment with one of the two ASOs they’ve developed. Each of these targets a different genetic variation, and having two drugs allows them to use their approach on a larger share of the HD population.
They’re testing the safety of these first two mutant Huntingtin lowering ASOs in HD patients in Canada, Europe and the US. Like the first Ionis/Roche trial, the goal of these studies is to determine whether these drugs are safe. If so, they would be subsequently tested for their ability to improve HD symptoms in larger studies.
We’ve also recently heard from Wave that they’re working behind the scenes on another ASO, targeting yet another variation in the HD gene. This third drug is not yet being tested in humans, but provides additional hope for folks who aren’t eligible for either of the existing Wave drugs. In 2019 we hope to hear preliminary updates about the human safety studies from the first two Wave trials, as well as more details on their program developing a third ASO.
What’s the P-T-C?
It’s not just ASOs in the huntingtin-lowering world. At the European Huntington’s Disease Network meeting in Vienna this fall, Anu Bhattacharyya, of PTC Therapeutics, updated the EHDN audience about exciting progress at her firm, which is also interested in Huntingtin lowering as a treatment for HD.
“PTC is developing what researchers call a small molecule, meaning a drug that can hopefully be taken as a pill, to lower levels of the Huntingtin messenger RNA. A few years ago this would have seemed like science fiction, but several companies have described experimental drugs where this approach appears to work.”
The approach taken by PTC is totally different than the ASO approach taken by Wave and Ionis/Roche. All the other programs involve injections into the brain or spinal column – worthwhile if they work, but much more invasive than we’d ultimately like to see. PTC is developing what researchers call a small molecule, meaning a drug that can hopefully be taken as a pill, to lower levels of the Huntingtin messenger RNA. A few years ago this would have seemed like science fiction, but several companies have described experimental drugs where this approach appears to work.
PTC is at the forefront of companies developing this new approach to interfering with specific messenger RNAs, and they have their eyes set on HD. They’ve developed a drug that reduces levels of the Huntingtin protein in cells. For the first time, at EHDN, Bhattacharyya revealed that these drugs also work in the brains of living mice – suggesting the drugs get from the stomach into the brain, which is a huge accomplishment.
What’s especially cool about this is that even if a drug taken as a pill reduces huntingtin in the brain by a smaller amount than an injected drug, it could still be really useful. A huntingtin-lowering pill could mean spinal injections of a more powerful drug could be needed every six or twelve months, say, instead of every one or two months.
PTC’s program is at an earlier phase than the Ionis/Roche and Wave programs, as it’s still being tested in animals. But it’s a genuinely exciting approach that might offer real benefits if it’s proven safe and effective in future trials. And PTC appears to be planning for the future – Bhattacharyya told the audience at EHDN that PTC had a goal of beginning safety studies in humans in 2020. Excitingly, PTC has a track record of success, with two approved oral medications for muscular dystrophy, another genetic neurological disease.
Voyager
At another conference this fall – the Congress of the European Society of Gene and Cell Therapy – we got another exciting huntingtin-lowering update from Voyager Therapeutics. Voyager is a biotechnology company focused on using gene therapy to treat brain diseases, including Huntington’s.
Gene therapy works very differently from ASOs or small-molecule drugs. Gene therapy relies on tiny, harmless, viruses to deliver new genetic information to cells – in this case brain cells. Viruses are very good at sneaking into cells, so clever researchers have figured out how to trick them into delivering beneficial things into different cells of the body.
In this case, Voyager’s team of researchers has built custom viruses that deliver instructions that tell brain cells how to make a special piece of RNA that seeks out the Huntingtin mRNA and destroys it. In effect, the virus reprograms cells to become factories to make a daily supply of a drug that works like the ASOs we discussed above.
This approach has a huge benefit, which is that the treatment only needs to be delivered one time. Once treated, the brain cell will theoretically produce the huntingtin-lowering molecule indefinitely. If safe and effective, this would obviously be better than getting monthly injections into the spinal fluid, or even taking a pill every day.
However, there are a few potential downsides to this approach. First – it might turn out to be unsafe in some way that we can’t foresee at this stage. And because the treatment isn’t reversible, we have to take extra caution with safety for gene therapy trials. Secondly, while it’s pretty easy to get these hacked viruses into most of the cells in a tiny mouse brain, it’s much trickier to do this to the 86 billion neurons in the human brain.
“Voyager’s team of researchers has built custom viruses that deliver instructions that tell brain cells how to make a special piece of RNA that seeks out the Huntingtin mRNA and destroys it. In effect, the virus reprograms cells to become factories to make a daily supply of a drug that works like the ASOs we discussed above.”
That’s what makes this update from Voyager scientists particularly interesting. They’ve reported on experiments conducted in monkeys, which have large complex brains that are much closer to our own. Voyager has developed surgical techniques that help the virus spread over very large portions of the monkey brain, both deep brain structures and the cortex – the wrinkly outer bit of the brain.
This is an especially important advance, because the deep brain structures targeted by Voyager are relatively poorly reached by ASO drugs, but play a key role in HD. Voyager’s experiments also reveal very good suppression of the monkey huntingtin gene – reductions of about two-thirds in deep brain structures, and about one third in the outer, cortical, brain cells. These are decent reductions, and one might hope that achieving similar results in human HD patients could provide some real benefits.
Like the Ionis/Roche trial, the path envisioned by Voyager is to reduce both the mutant and the normal copies of the HD gene. Given this, and the fact that their therapy can’t be shut off after it’s delivered, this approach demands a super-cautious approach, which they appear to be taking with all these monkey studies.
Take home
There is a lot of justified excitement in the HD community about the ongoing Ionis/Roche/Genentech study. Everyone, including HDBuzz, is pulling for this study and has high hopes that it will provide some benefit to HD patients. But, as these recent advances show, it’s not the only game in town. There are other approaches to Huntingtin lowering that are not quite as far along, but offer large potential benefits.
So to recap: there are two ongoing trial programs with huntingtin-lowering ASOs in patients. One targets both copies of the HD gene (Roche/Genentech), has passed the safety test, and will soon be tested for efficacy. The other targets only the mutant gene (Wave), and is currently being tested for safety. Behind that, we have single-shot gene therapy trials undergoing extensive safety studies in preparation for human trials (Voyager and others), and a really novel small molecule approach (PTC).
Supporting multiple shots on goal doesn’t just increase the chance that one will succeed – it also offers the chance that more than one might work. That offers a tantalising future where combinations of drugs might be used to produce the maximum benefit for the lowest possible risk. Combination approaches have proven successful for other diseases like HIV, cancer and diabetes. When it comes to drugs in development, and drugs that work, more is more.
Learn more
Sources & References
For more information about our disclosure policy see our FAQ…


