
Huntington's Disease Therapeutics Conference 2017 – Day 1
HDBuzz summarises all the science from the 2017 Huntington's Disease Therapeutics Conference in Malta – day 1

Hi everyone! It’s the annual Huntington’s Disease Therapeutics Conference in Malta. Around 350 scientists from round the world all working on HD are gathered here to discuss the latest research developments. We’re reporting live via twitter and aggregating our updates into daily articles.
Big news from big data
The first speaker is Leslie Jones from Cardiff University, who studies how genetics influences HD. Jones is interested in how genetic variations causes people with the HD mutation to have symptom onset earlier or later than expected. She is part of the “GeM-HD” consortium of investigators that published a landmark study in 2015. Genetic variations which modify the age of onset of HD work similarly in people with other diseases called “spinocerebellar ataxia”. Like HD, some forms of SCA are caused by an expanded “C-A-G” sequence (in different genes). These long, repetitive, stretches of “C-A-G” are difficult for cells to copy accurately. As a result, long CAG repeats tend to get longer, because of slip-ups in the process of copying and repairing DNA. The major finding of the GeM-HD study is that mutations in DNA repair genes influence the age at which HD symptoms occur. Scientists working on HD are suddenly having to learn a lot about the process of DNA repair!

Davina Hensman Moss (UCL) is a key member of the group studying how genetic variations influence the age of HD symptom onset. We tend to talk about “onset” of HD, but the progression of HD symptoms is a long, slow, process. Moss is using information from the TRACK-HD study to understand how this progression happens. Information provided by the TRACK-HD study allowed Moss to build a sophisticated computer model of HD symptom progression. She then asked a very clever question – do people whose HD progresses quickly (or slowly) have any correlated genetic differences? They looked for variations throughout the entire genome of the TRACK-HD participants whose HD course was unusual. They discovered genetic variations outside the HD gene which lead to alterations in the rate of progression of HD symptoms. Moss observes that variations in DNA repair genes changes the rate of HD progression. This is overwhelming evidence that the process of DNA repair is important in HD, though the details are not yet clear. The clear message from these studies is that helping cells copy the CAG repeats in the HD gene accurately is critically important. This finding is only possible thanks to thousands of HD Community members participating in studies and donating DNA. These genetic studies were done using technology that didn’t exist when most participants donated their DNA for research.
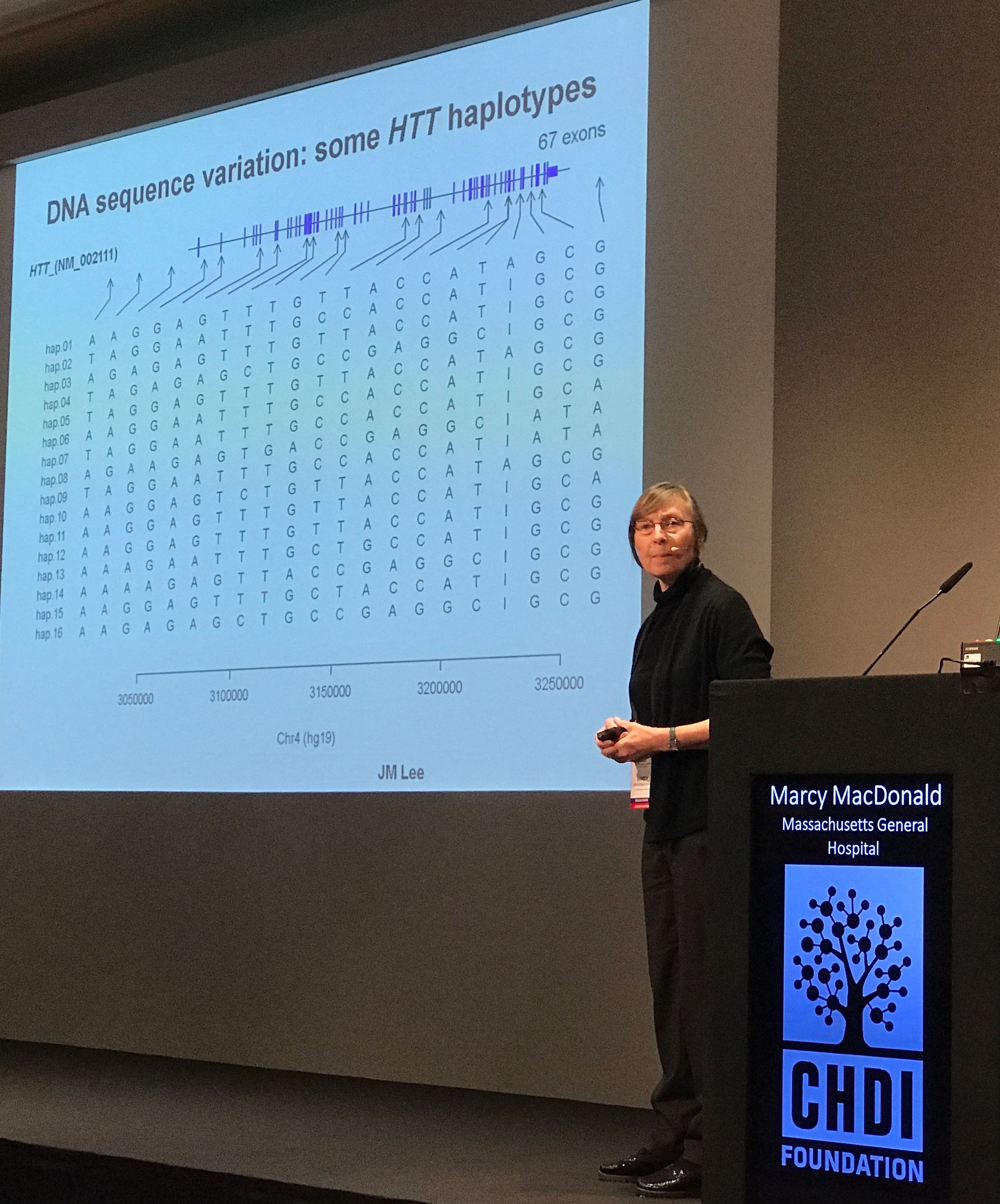
Chris Kay (UBC) addresses the conference about a different type of genetic variation – changes in the sequence of the HD gene itself. Kay is mapping tiny spelling variations in the HD gene, outside the CAG region. Kay has found a number of variations that are much more common in HD genes with CAG expansions than those without. This helps us understand the historical origin and world-wide distribution of the HD-causing CAG expansion. It also provides very interesting targets for researchers hoping to selectively reduce levels of the mutant HD gene.
Christian Neri from Inserm studies a protein called FOXO and its role in HD. FOXO helps brain cells cope with ‘stress’, which is what scientists call anything that makes it harder for cells to do their thing. FOXO controls lots of coping mechanisms cells can use to survive and cope with stress.
Matthias Mann is a pioneer in the field of “proteomics”. This technology enables scientists to accurately measure the levels of thousands of proteins at one time. Genes are exciting, but proteins do most of the work of the cell, Mann’s group has developed remarkable tools for measuring them. Mann’s group is now beginning work in HD, applying their methods to try and find new ways to track HD progression.
“This finding is only possible thanks to thousands of HD community members participating in studies and donating DNA.”
Public enemy number one: mutant huntingtin
Marcy MacDonald (MGH) has spent her career studying variation in the HD gene. MacDonald is a key member of the GeM-HD consortium, who are mapping genetic variants that influence the progression of HD symptoms. They’ve discovered a brand new variant that modifies HD progression using DNA from more than 7,000 individuals. The new variant is very rare – only by pooling information from thousands of volunteers was the team able to make the discovery.
Darren Monckton (U. Glasgow) is interested in the CAG repeat of the HD gene. We’ve known since 1993 that expansion of a CAG repeat in the HD gene causes every case of HD. More recently, Monckton and other researchers are understanding that CAG repeats can lengthen during the lifetime of a cell. Monckton’s team is using new sequencing technologies to sensitively measure the length of the HD CAG tract in 1,000’s of HD patients. They hope that finding rare variations in the organization of the CAG tract will help us understand how CAG tracts get longer. Monckton found that DNA repair genes Dr Hensman Moss talked about earlier, influence the spread of CAG repeat sizes seen in a person’s cell. (Sidebar: the CAG count in a genetic test result is actually the average count – different cells contain slightly different numbers) One theory about why some people progress rapidly is that the CAG count might increase in vulnerable brain cells. Geneticists call this ‘somatic instability’ of the CAG repeat. Understanding somatic instability is a key mission because if we could reduce it, we might help brain cells survive longer Reducing somatic instability might also prevent the tendency of the CAG count to grow from one generation to the next.
Kevin Weeks (UNC) is interested in RNA – the chemical intermediate ferrying information from DNA to make proteins in cells. RNA is often thought of as a linear string of letters copied from the DNA, but RNA folds up into complex shapes with important roles. Weeks’ lab is building really precise models of the 3D shape of the RNA made from the HD gene. It changes as the number of CAG repeats in the HD Gene increases – more CAGs lead to big changes in 3D RNA structure. These changes in shape provide a unique target for Week’s team to try and target – and maybe reduce – RNA levels for the mutant gene. This opens up a new way to stop cells from making mutant huntingtin protein, the most likely culprit for making cells sick in HD.
While HD most frequently occurs in adulthood, Mark Mehler (Albert Einstein College of Medicine) focuses on very early changes. He finds subtle changes in brain development in mice carrying very long CAG expansions in the HD gene. These changes may make brain cells more vulnerable in adulthood.
Alberto Ruzo (Rockefeller) is also interested in the process of development – the process by which a fertilized egg becomes an adult. During development, special cells called “stem cells” divide to eventually produce all the cell types found in an adult body. Ruzo has created new stem cells which are genetically identical, except for an increasing amount of CAG repeats in the HD gene. This allows them to study the process by which dividing stem cells grow into the complex shapes which give rise to adult organs They observe changes in these shapes in cells with longer CAG repeats.
For more information about our disclosure policy see our FAQ…


