HDSA’s Q&A on the latest huntingtin-lowering update from Ionis and Roche
Our friends at @hdsa have produced this really good Q&A document about the latest huntingtin-lowering news.

Earlier this week, Ionis Pharmaceuticals put out a press release after presenting data from the HTTRx (RG6042) Huntingtin-lowering clinical trial program at a scientific conference. Ionis’ partner Roche also released a community statement. Our friend at Huntington’s Disease Society of America, Dr Leora Fox, has prepared this Q&A in response to community questions received after the release. We liked it so much, we decided to reproduce it here, by kind permission, for the benefit of HDBuzz readers.
What did the press release say?
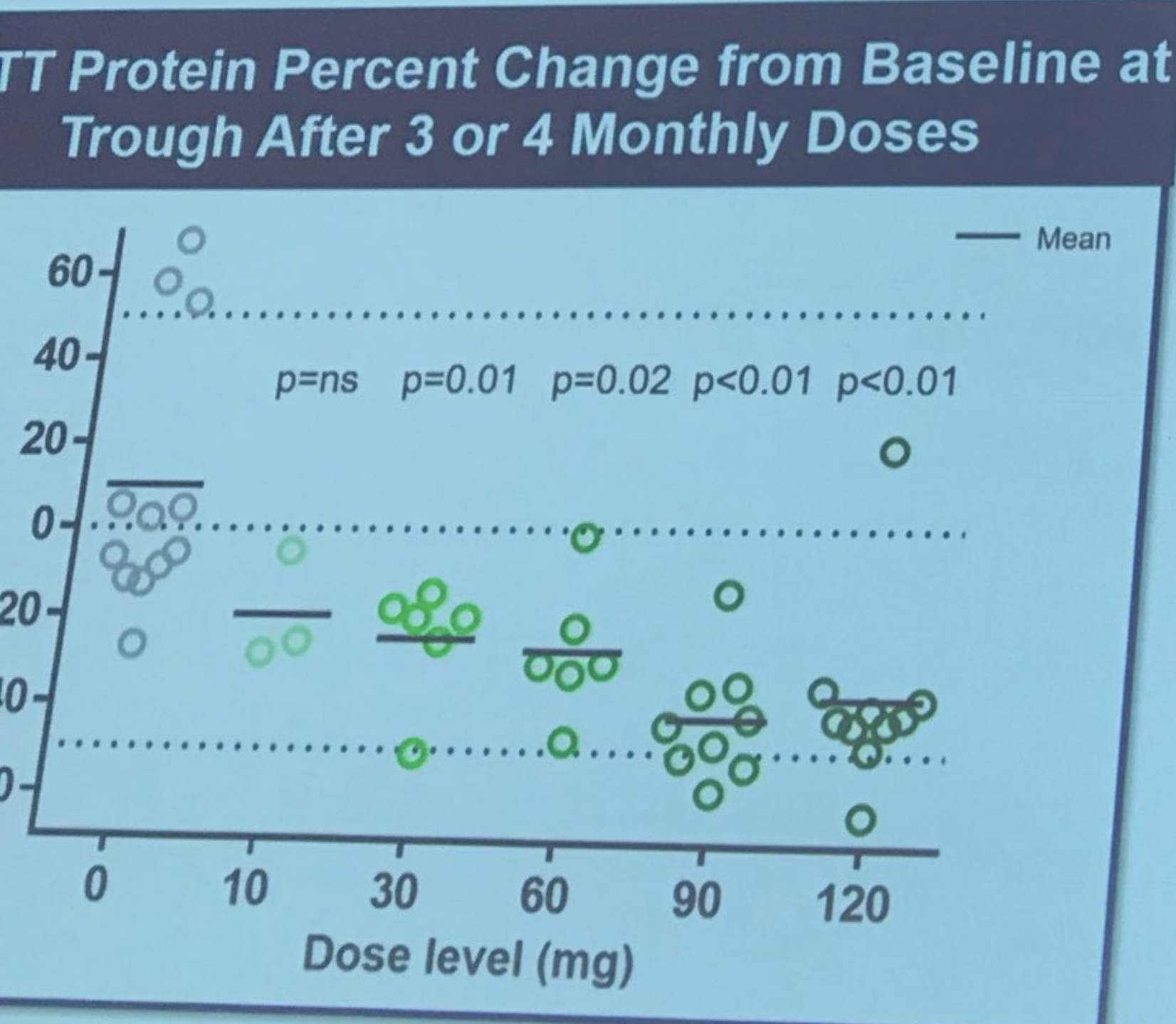
Mainly, it restated what we already know: the drug is safe, and it lowered levels of huntingtin, the protein that harms brain cells in HD. The newest information, presented on April 24th, is that overall, participants with lower huntingtin levels also did a bit better on clinical exams that test HD symptoms. However, the study was only designed to be sure of safety, not efficacy, so a larger trial still needs to be conducted.
So are they saying that the drug worked?
It’s not possible to draw that conclusion right now. All we know is that it’s safe and landed on the genetic target it was designed to hit. The clinical findings shared by Ionis are exploratory – the trial only involved 46 participants in total, which is not enough to be sure it could help with symptoms. Even though the overall conclusions are very promising, the statistics have not yet shown a clinical benefit.
What’s next? Does this knowledge change anything going forwards?
Nope. Ionis is passing the torch on to Roche Pharmaceuticals, a larger company that is invested in the HD community. In the United States, the company is called Genentech. An experienced international team has been assembled and is tasked with the planning and coordination of a global Phase 3 clinical trial. You can read their most recent message to the community here. In the meantime, we’re encouraged that the analysis of the Phase ½ data continues to show promising results. We’re also really excited that these results are being given the spotlight at international research conferences. This will positively impact families by raising awareness of HD among medical professionals worldwide.
If it’s so promising, what’s the holdup?
Ensuring that drugs are safe and effective is a time-consuming and heavily regulated process. Roche-Genentech has a lot of work to do, including designing the trial with care, identifying the medical teams and facilities that will take part, making sure that professionals are equipped and trained to administer the drug, producing the drug itself, and sorting out funding and regulations at different agencies worldwide. All of these steps require planning, paperwork, and patience. Rest assured that there are smart and compassionate minds involved, including HD patients and families, working to bring this drug to the clinic as quickly as possible.
Can I sign up to be in the trial, or put my name on a list?
Unfortunately, no. The way that clinical trials usually recruit is through existing doctor-patient relationships, where the doctor decides if a patient might be eligible, and makes a referral. That’s one reason why HDSA encourages people [in the USA] to see an HD expert at an HDSA Center of Excellence or other HD clinic with a research interest, and to join Enroll-HD.
There’s been an outpouring of folks who are ready to take a big leap and participate. The trial is not up to that stage yet, and HDSA is not in control of any aspect of the design, participation, or eligibility. There will be many fewer “spots” than willing participants, which will understandably lead to some disappointment. We do not encourage people to consider a big life shift (like moving) based on their desire to participate in a trial. However, we are thrilled about the community’s engagement and we’ll continue to provide and interpret any new information we hear.
There may be other research opportunities near you, as well – check out HDTrialfinder for details.
What is HDSA doing to support the trial and the community?
HDSA staff are working very closely with members of the Roche-Genentech team to ensure timely and proper information about this trial will be shared with the community as soon as it becomes available. HDSA is also a founding member of the Huntington’s Disease Coalition for Patient Engagement (HD-COPE), which helps industry teams connect directly with patients and families. HD-COPE will help companies like Roche to make sure that trial planning, measurements, and participation requirements seem reasonable to the real experts – people with HD and their loved ones.
Finally, we continue to provide trial details and contact information for participating in open research studies on HD Trialfinder. Because the upcoming trial has not yet begun, it is not listed there.
When do you think this drug will be available?
That’s extremely difficult to answer. Trials can take several years, and a lot of care must be taken with this one, because it’s so important and so different from drugs that address just the symptoms of HD. We’d love to be able to say there’s a definite timeline, but this is uncharted territory and we simply don’t know. The first step is to see whether it can improve symptoms or slow down HD, and that’s the focus of the Phase 3 trial.
What can I do right now?
Make sure you’re under regular care with a doctor or team who has experience with HD, particularly one with a research interest (like an HDSA Center of Excellence or university HD clinic). Follow the news at HDBuzz, on HDSA’s weekly research blog, or on HDSA’s social media pages. Check out HD Trialfinder for research opportunities. Above all, take care of yourself and those around you!
In the fight against HD, family is everything.
Learn more
Sources & References
For more information about our disclosure policy see our FAQ…


