
Huntington's Disease Therapeutics Conference 2016 – day 1
Proteins and DNA – Jeff and Ed report from day 1 of the 2016 #huntingtonsdisease therapeutics conference

If it’s February, that means the the world’s leading scientists are converging on Palm Springs for the annual HD therapeutics conference!
Monday evening – updates from CHDI Foundation
Before the meeting officially starts, staff from the CHDI Foundation are giving updates about the exciting science happening there.

Tom Vogt, vice president of Discovery and Systems Biology at CHDI, is updating the audience on CHDI’s efforts to better understand HD. Vogt reminds the audience of a huge study published this year that revealed genes which modify HD age of onset. These genes might help us better understand how HD happens, but also provide important “targets” for drug hunters. To make this breakthrough, the research team required DNA samples from literally thousands of HD patient volunteers.
Another huge study, this one of HD mice, has resulted in huge amounts of data about how the HD mutation impacts different tissues. Importantly, these new huge sets of data have been made available online to any scientist around the world who wants to study them. Any researcher who wants to access both the mouse and human HD data can find it here: https://www.hdinhd.org/
Celia Dominguez, VP of chemistry at CHDI, updates the audience about the drug development efforts happening at the foundation. Studying HD patients, and animal models, is important but ultimately we need chemists to make drugs that we can deliver to people. Dominguez and team develop not only experimental drugs, but other novel chemicals that expand what HD physicians and scientists can do.
Dominguez’s team is looking for drug-like chemicals that reduce levels of the mutant HD protein – the cause of every case of HD. Dominguez updates the audience on CHDI’s efforts to develop drugs that help cells deal with DNA damage, which accumulates in HD. Data from mice suggests that modulating how cells handle damage to their DNA can improve HD-like symptoms. But mice aren’t people, and researchers are developing new drug-like chemicals in hopes of mimicking the effects seen in mice.
Finally, Dominguez reveals chemists are working to devlop a chemical that would enable scientists peer into the brains of HD patients. A chemical tag that would let scientists measure levels of the harmful HD protein in living patients’ brains! Dominguez’s team is using animal and human tissue studies to probe whether this new chemical does what it’s designed to do. The early studies look very promising, and are now proceeding rapidly to early trials in humans with the HD mutation.
If this works in humans, gene silencing trials would have a powerful new means of demonstrating efficiency
Prof Cristina Sampaio gives a comprehensive update on CHDI"s clinical programs including Enroll-HD which gives a platform for trials. CHDI is keen on designing ‘well-powered’ studies – that is, they have enough participants to answer the question they’re looking at. CHDI also aims to make data so the whole community can access it.
Tuesday Morning – the Huntingtin protein
The 2016 HD therapeutics conference starts with a session focusing on Huntingtin – the protein made from the HD gene. Professor Elena Cattaneo reminds the audience that the HD gene is ancient – first appearing over 800 million years ago! While only humans get Huntington’s Disease, we can find the gene in sea urchins and slime molds. It’s is hard to study, in part, because it’s is about 10 times larger than the average human gene.
Cells use genes as recipes to construct ‘proteins’ that do work in the cells. So we have an HD gene, and also HD protein (huntingtin). It’s likely that the brain cell damage that causes HD is a result of the bad effects of the protein, not the gene itself.
“Damage to DNA must be repaired, but it seems like this normal process goes awry in the face of expanded CAGs in the HD gene.”
This morning’s session is focused on better understanding this ancient, large and important protein. Better understanding of the protein that causes HD might help us design better therapies for HD in the future.
Ralf Langen, USC, is using a range of advanced imaging techniques to try and take a picture of the HD protein. Langen’s team is working with purified bits of the HD protein, and can infer things about how it’s shaped using sophisticated tools. Proteins in cells do their job thanks to their unique shapes. So work like Langen’s is critical to understand what the HD protein does. And, perhaps most importantly, Langen is attempting to understand how the structure of the HD protein is changed by the HD mutation. One of the funny things about the HD protein is that when it’s mutated it clumps up inside of cells. Some scientists believe these clumps, rather than individual HD proteins, could cause problems for brain cells. Another effort in Langen’s lab is understanding how the structure of an individual HD protein changes shape as it clumps up.
Hilal Lashuel is another researcher working to understand the shape of the HD protein. In particular, Lashuel is interested to understand how the shape of the HD protein responds to “post-translational modifications” (!). Proteins, after being made in a cell, get decorated with different chemical tags that change the shape of the protein. Since, for proteins, shape determines function, these little tags tweak the function of the protein in the cell. Why do we care about chemical tags on the HD protein? We know from mouse studies that specific tags make the HD protein less toxic. Lashuel’s team has developed chemical techniques to synthesize large amounts of the HD protein with any specific chemical tag desired. This enables researchers to study the impact of any combination of tags on the HD protein’s shape and function.
Long-time HD researcher Scott Zeitlin is also trying to understand the bit of the HD protein that’s mutated in Huntington’s disease. Rather than using test tubes and chemistry, Zeitlin uses mice in which little bits of HD gene have been deleted. The simplest way to study what a bit of DNA does is to break it and see what happens! Surprisingly, Zeitlin finds that he can delete important regions of the HD gene without having a major impact on mice. Zeitin is now trying to understand if deleting important bits of the HD gene makes HD-like symptoms in mice worse
Fred Saudou from INSERM is now talking about his work on what huntingtin protein actually does in cells – still a bit of a mystery. The predominant theory is that the mutation causes the protein to ‘gain’ behaviours or functions that are toxic to neurons. Saudou: huntingtin is a big protein with spring-like regions that might look like several slinkies strung together. One well-known thing huntingtin does is help to move chemicals around within our neurons. Huntingtin helps to deliver a helpful chemical called BDNF from the surface to the deep structures of the brain. BDNF is needed to keep cells in the striatum healthy but in HD, delivery of BDNF is reduced and cells in the striatum die. Saudou: The failure of BDNF delivery in HD might be because the mutant protein can’t supply energy to the cell’s molecular motors. Saudou used a protein-snipping enzyme borrowed from a virus, to see what happens when huntingtin protein is cut in different places. Surprisingly, Saudou found that not one but TWO cuts were needed to make the mutant huntingtin protein toxic to cells.
This is interesting – we previously thought that just 1 cut carried out by cellular snipping enzymes, in the right place, was enough. Saudou found that the tail end of the protein – the ‘C terminus’ was more important than previously thought. It seems certain fragments of the C-terminus are poisonous in themselves, even when the protein isn’t the mutant form. Interesting! But non-mutant protein isn’t poisonous, so there must be some interaction between the C terminus and the bit altered by the mutation. Saudou found that the C-terminal bit of huntingtin interferes with another protein called dynamin 1 which is important for transport. Saudou’s work to study the whole protein and the bits it’s snipped into continues.
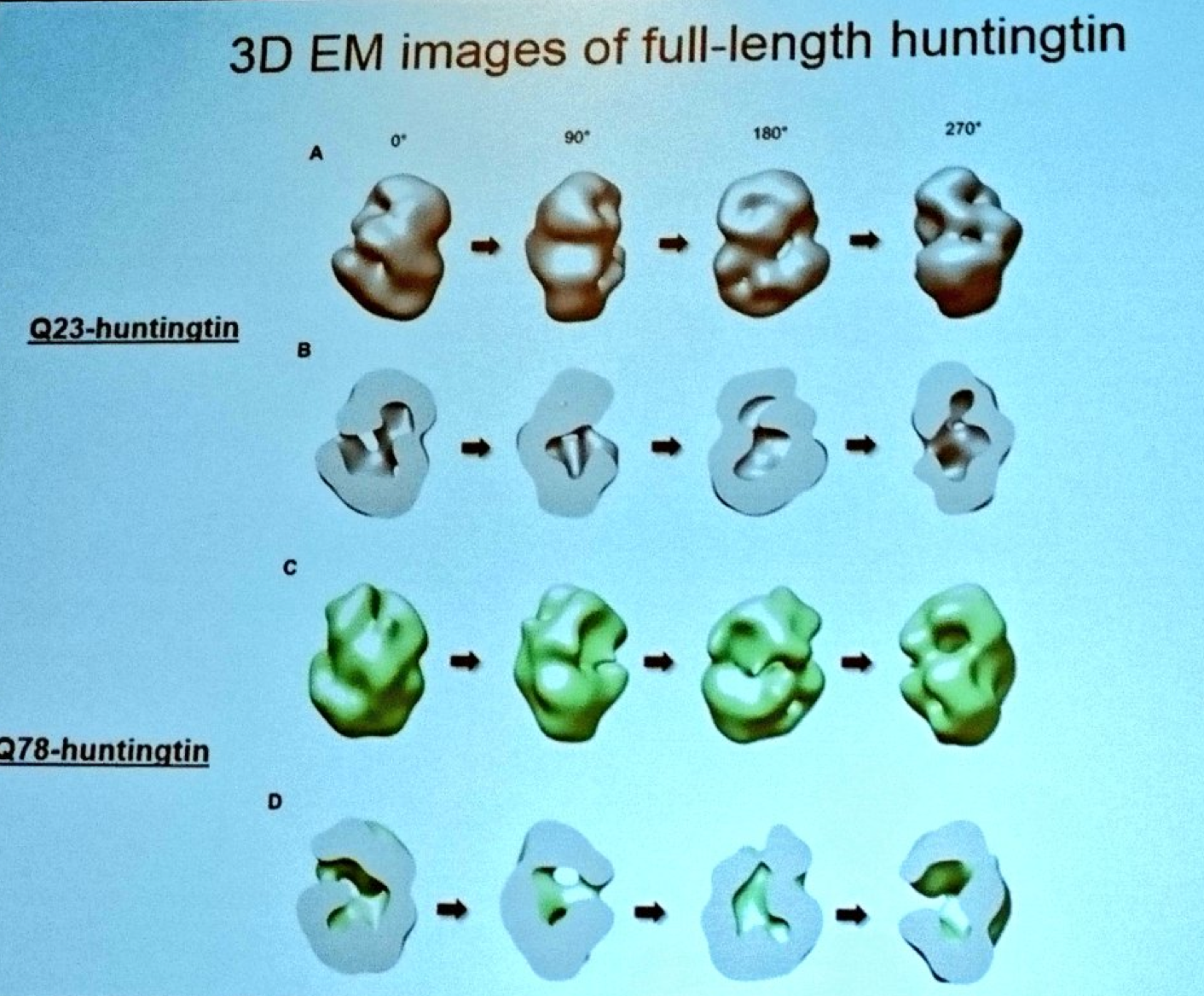
Next we hear from Ihn Sik Seong from Massachusetts General Hospital. Seong has developed techniques to purify the complete huntingtin protein so he can study its structure. Using a super powerful electron microscope, Seong has developed a model for the appearance of the huntingtin protein. Because he can make both normal and mutant huntingtin, Seong can see how the overall shape of the protein is changed by the mutation. Seong’s modeling suggests that the huntingtin protein is folded up on itself, forming a hollow shape like a tennis ball. Bringing things full circle, Seong is working to understand how chemical tagging (post-translational modification) modifies huntingtin
Great session! All this basic science about how the HD mutation impacts huntingtin structure empowers the next wave of discoveries!
Tuesday afternoon – DNA and RNA
This afternoon we’ll continue the theme of basic science, but switch from talking about proteins to talking about DNA. Stay tuned!
Steve Horvath, UCLA, is interested in chemical changes in DNA that happen in aging. One of these chemical changes is so reproducible that Horvath has shown it can be used like clock to predict the age of a cell. Using this genetic clock, Horvath finds that different parts of the body “age” at different rates. So what about brain samples from people with brain diseases, like HD? Do brains of HD patients age faster than people without HD? Yes – Horvath finds that HD brains look older than they should, using his new genetic clock method.
Karine Merienne is studying a process called “transcription”, the process by which cells decide which genes they need to make, in HD. Every cell in the body has a copy of each of the approximately 25,000 genes we all inherited from our mom and dad. Brain cells need brain genes, and skin cells need to make skin genes. “Transcription” is the process of turning on the right genes. The pattern of gene activation is altered in HD patient brains, and Merienne is using HD mice to try and understand why. Merienne has found a specific process seems to go wrong in HD cells, which might help explain some of the changes in transcription. An experimental drug that improves this specific transcriptional process seems to protect the memory performance of HD mice.
Vanessa Wheeler studies how the mutation that causes HD worsens in specific tissues during aging. Every person with HD has inherited a specific change in their DNA, an expansion of the DNA sequence “C-A-G” in their HD gene. In HD patients, the CAG repeat is always longer than normal – repeated more than 36 times. In general, a longer CAG repeat is associated with having HD symptoms at an early age, though there’s a lot of variation across people. Wheeler’s lab is interested in a strange phenomenon – in some tissues of the body, the CAG repeat tends to get longer as someone ages. The striatum, the part of the brain that is most affected by HD, is a part of the body where CAG expansion happens the most.
Wheeler would like to understand why the CAG repeat length in certain parts of the body has a tendency to grow over time. Wheeler’s team has found that a process called DNA repair causes the expansion of the CAG repeat region in the HD gene. Damage to DNA must be repaired, but it seems like this normal process goes awry in the face of expanded CAGs in the HD gene. A recent study of HD patients suggests altered DNA damage responses across people might help explain variations in age of onset of HD. Wheeler is conducting extensive experiments in new mice she’s developed to understand better how this “CAG expansion” process happens.
Next Laura Ranum of University of Florida is talking about a new and controversial idea. Think of the word CART. If you’re told to read 3 letters from the start, you’ll get CAR. But if you read 3 letters from the 2nd, you get ART. Something similar can happen when cells read DNA. Most of the time the reading starts at the beginning but occasionally things slip and the DNA gets read out of sequence, producing RNA messages or even proteins that are unexpected. Occasionally the DNA is even read backwards producing strange ‘hidden’ RNA messages or proteins. Ranum discovered this weird DNA reading mechanism & called it RAN transcription (coincidentally it sounds like the start of her name!).
In HD, we know that reading the DNA in the NORMAL way produces the mutant huntingtin protein, and we know that is harmful to neurons. The question is whether mutant huntingtin is the only toxic product of the expanded gene or whether RAN transcription might produce toxic RNA or other harmful proteins through weird DNA-reading. Ranum’s team looked at brain samples from HD patients who had died, and found some of these weird proteins in some areas. Finding these RAN proteins in the brain is a start, but doesn’t necessarily mean they are causing harm. In cells grown in the lab, Ranum has shown some RAN proteins can be harmful- though that doesn’t necessarily translate to whole brains.
Ray Truant (Mcmaster University) is interested in understanding what the huntingtin protein does normally, and how the mutation that causes HD changes this. Like many of this morning’s speakers, Truant is especially interested in little chemical tags that decorate the huntingtin protein. Truant uses robotic microscopes and sophisticated software to automatically analyze the effects that drugs have on HD cells. Using these techniques, Truant’s lab follows the huntingtin protein around the cells exposed to different kinds of stress. Truant has discovered that a chemical called kinetin helps keep mutant HD cells healthy.
When HD mice are treated with kinetin, some HD-like symptoms are improved, supporting the idea that this molecule might be helpful. Truant’s lab has also done extensive experiments which suggest that the Huntingtin protein might be involved in DNA damage. His hypothesis is that kinetin helps the mutant huntingtin protein do it’s job better in responding to DNA damage. Truant links some of the talks discussed already today – hints that the huntingtin protein might play some role in DNA damage. Truant proposes to study kinetin as a potential therapy in HD.
For more information about our disclosure policy see our FAQ…


